A microwave radiation-assisted synthesis method of n-methyl-2-cyano-3,4-disubstituted pyrrole compound
A technology of disubstituted pyrrole and trimethylcyanosilane, which is applied in the field of organic intermediate synthesis, can solve the problems of low reaction efficiency, low atom utilization rate, and cannot be applied in industry, and achieves simple reaction conditions, environmental protection and green reaction conditions. , the effect of improving atomic utilization and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
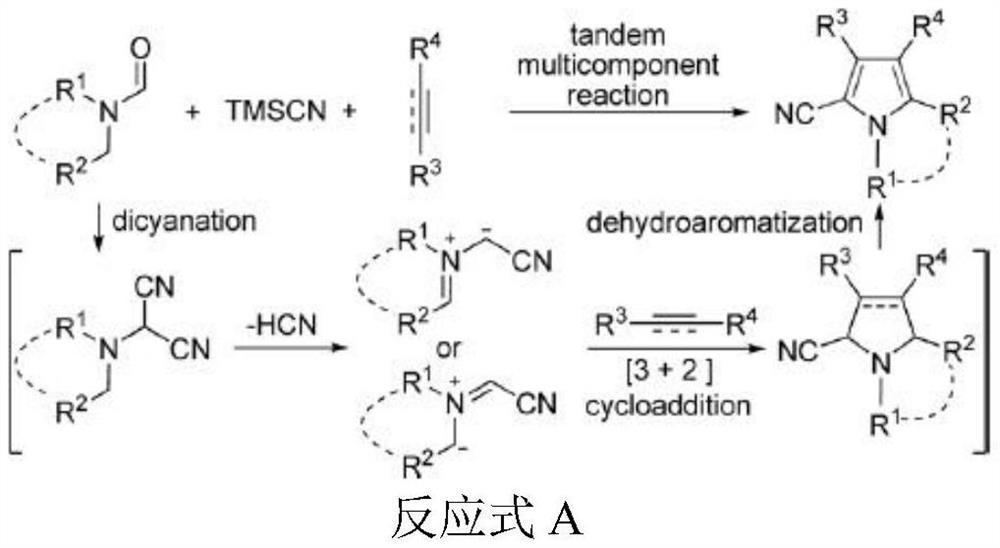
[0051]The following Examples 1 to 3 are reacted in the following reaction equation:
[0052]
[0053]The specific steps are: in a 50 ml round bottom flask, an alkyne (30 mmol), trimethylthilane (30 mmol, 2.97 g), N, N-dimethylformamide (30 mmol, 2.19 g), iodine Sodium (3 mmol, 0.45 g), the resulting mixture was stirred at 300 W in the microwave reactor, and the reaction was stirred at 10 ° C for 10 minutes. After completion of the reaction, 30 ml of ethyl acetate was dissolved, saturated brine wash solution, dispensing, and concentrated in vacuo, vacuum drying calculation weight.
Embodiment 1
[0055]raw material:Target product:
[0056]1, 4-Dimethyl-3-Phenyl-1H-Pyrrole-2-Carbonitrile:
[0057]Yield: 92%.
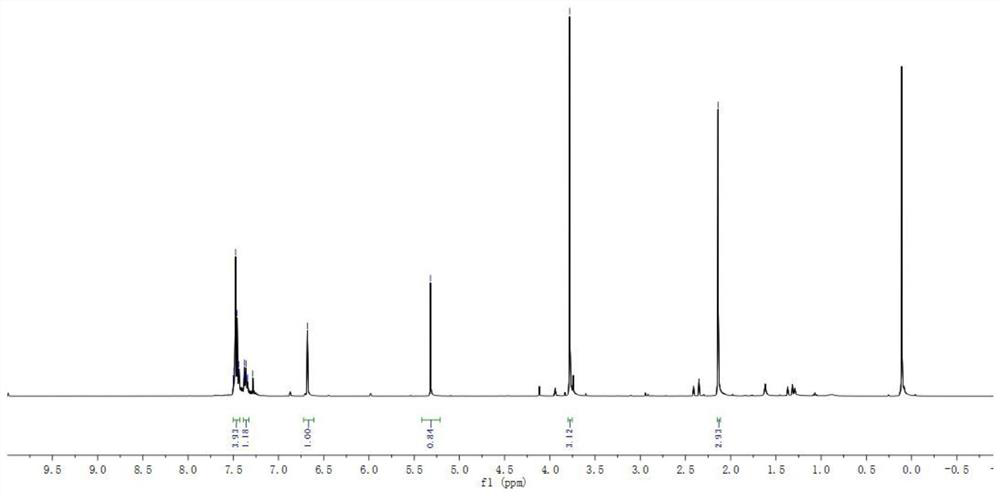
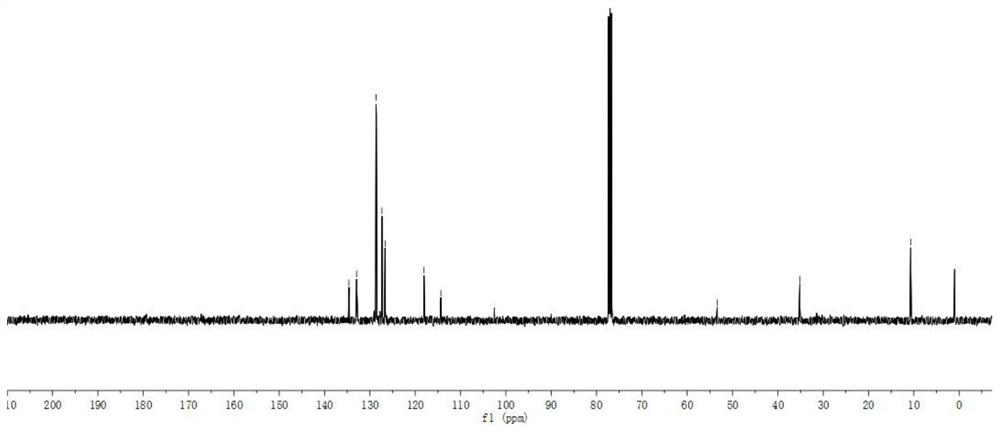
[0058]1H NMR (500MHz, CDCL3: 7.50-7.44 (m, 4H), 7.39-7.34 (m, 1H), 6.88 (S, 1H), 5.32 (S, 1H), 3.78 (S, 3H), 2.14 (S, 3H).
[0059]13C NMR (125MHz, CDCL3: 134.6, 132.9, 128.6, 128.6, 127.3, 126.6, 118.1, 114.34, 53.4, 35.2, 10.7.
[0060]HRMS (ESI) Calcd for C13Hide13N2[M + h]+: 197.1079, Found 107.1081.
Embodiment 2
[0062]raw material:Target product:
[0063]Yield: 93%.
[0064]1-Methyl-3,4-Diphenyl-1H-Pyrrole-2-Carbonitrile
[0065]1H NMR (500MHz, CDCL3Δ7.35-7.27 (m, 5H), 7.26-7.22 (m, 3H), 7.18-7.13 (m, 2H), 6.92 (S, 1H), 3.85 (S, 3H).
[0066]13C NMR (125MHz, CDCL3Δ133.7, 133.4, 132.4, 129.3, 128.7, 128.6, 128.5, 127.7, 126.7, 126.4, 124.5, 114.3, 104.3, 35.7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
| power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com