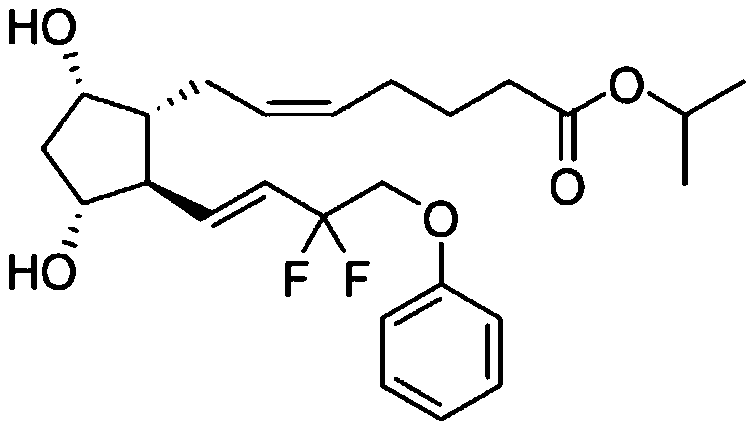
Tafluprost eye drop agent and preparation method thereof
A technology of tafluprost and eye drops, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of poor API solubility and stability, complex prescription composition, impurities, etc. Wait for the question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Weigh 100g of methyl cellulose (MC), add it into a 10L predissolved tank containing 4L water for injection, use a heating mantle outside the predissolved tank (hot water can be passed through, cold water can control the temperature in the tank), stir at 10°C to completely dissolve and clarify, Then weigh 0.2 g of tafluprost and add it into the pre-dissolved bucket, and stir at 10° C. to dissolve completely. Weigh 2g of lauryl alcohol and dissolve it in 1L of 10°C water for injection, stir to disperse evenly, pour into the tafluprost aqueous solution prepared above, and continue to stir until the solution is clear. Transfer the solution in the pre-dissolved bucket to a 25L liquid mixing tank, add water for injection at about 10°C to make the total solution reach 18L, test the pH value of the solution to 5.0, continue stirring in the liquid mixing tank and maintain the temperature at 10°C , use the prepared 1mol / L sodium hydroxide aqueous solution to adjust its pH value t...
Embodiment 2
[0052]Weigh 600g of carboxymethylcellulose sodium (CMC-Na), add it into a 10L predissolved tank containing 6L water for injection, use a heating mantle outside the predissolved tank (hot water can be passed through, cold water can control the temperature in the tank), and stir at 20°C Completely dissolve and clarify, then weigh 0.5g of tafluprost and add it to the pre-dissolve bucket, and stir at 20°C to dissolve completely. Weigh 8g of oleic acid and dissolve it in 3L of 20°C water for injection, stir and disperse evenly, pour it into the tafluprost aqueous solution prepared above, and continue stirring until the solution is clear. Transfer the solution in the pre-dissolved bucket to a 25L liquid mixing tank, add water for injection at about 20°C to make the total solution reach 18L, test the pH value of the solution to 7.2, continue stirring in the liquid mixing tank and maintain the temperature at 20°C , use the prepared 1mol / L sodium hydroxide aqueous solution to adjust it...
Embodiment 3
[0054] Weigh 400g of hydroxypropylmethylcellulose (HPMC), add it into a 10L predissolved barrel containing 5L water for injection, use a heating mantle outside the predissolved barrel (hot water can be passed through, cold water can control the temperature in the canister), and stir at 15°C to dissolve completely After clarification, weigh 0.4g of tafluprost and add it into the pre-dissolved bucket, and stir at 15°C to dissolve completely. Weigh 5g of pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)]propionate and dissolve it in 2L of 15°C water for injection, stir and disperse evenly, and pour it into the above-prepared taflur In the plain aqueous solution, continue to stir until the solution is clear. Transfer the solution in the pre-dissolved barrel to a 25L liquid mixing tank, add water for injection at about 15°C to make the total solution reach 18L, and test the pH value of the solution to be 5.8. When the liquid mixing tank continues to stir and the temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com