Imaging probe, and preparation method and application thereof
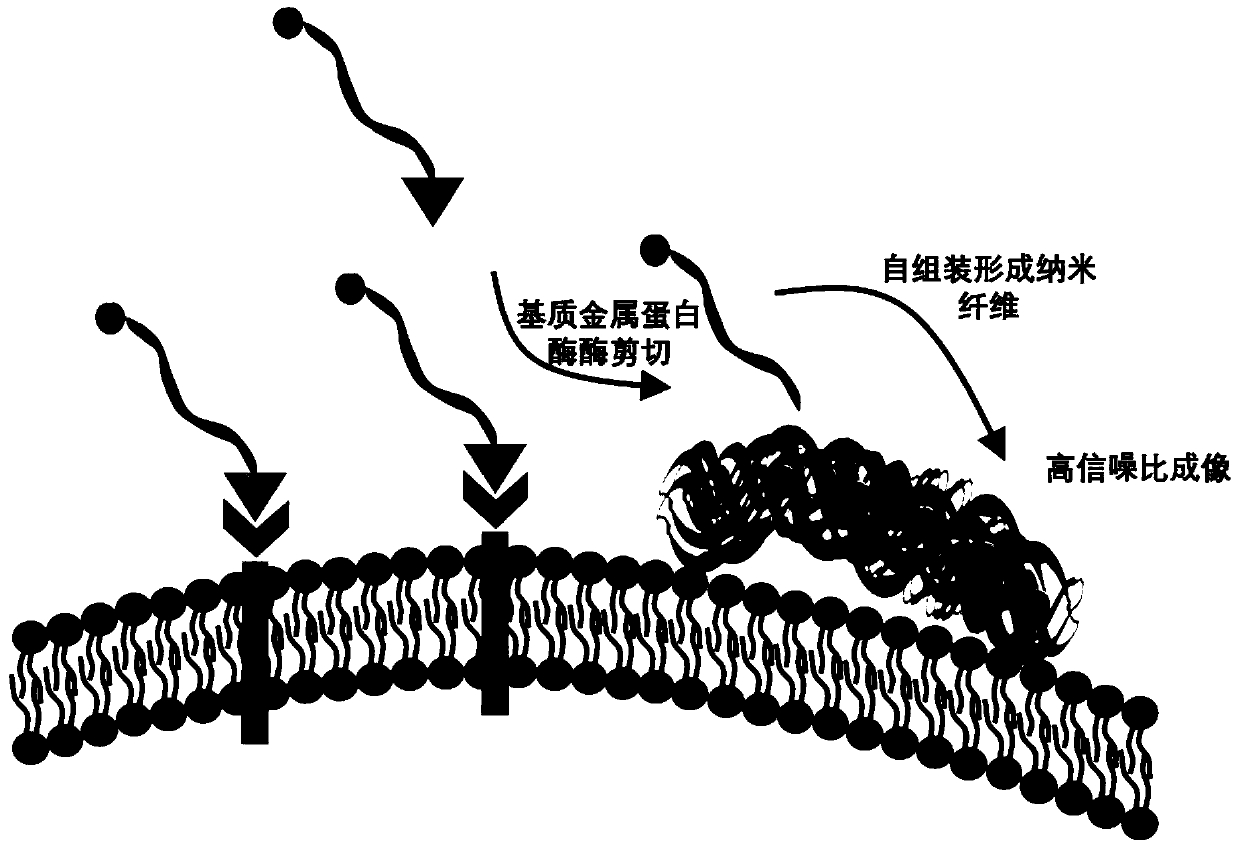
An imaging probe and self-assembly technology, applied in the fields of tumor imaging and medicine, can solve problems such as limiting the application of intraoperative navigation technology, achieve long-term retention effects, increase fluorescence signal-to-noise ratio, and break through low fluorescence signal-to-noise ratio Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] This embodiment provides an imaging probe RGDRDDRDDPGYLGFFC-Cy, whose structural formula can be represented by formula I, and the probe is prepared by polypeptide solid-phase synthesis.
[0064] Utilize the step that described probe is synthesized by polypeptide solid-phase synthesis method as follows:
[0065] (1) Synthesis of the polypeptide sequence RGDRDDRDDPGYLGFFC (hereinafter referred to as "polypeptide 1")
[0066] Experimental instruments and materials:
[0067] Dimethylformamide (DMF), piperidine, Wang resin, dichloromethane (DCM), ninhydrin reagents (ninhydrin, vitamin C, and phenol), benzotriazole-N,N,N' ,N'-tetramethyluronium hexafluorophosphate (HBTU), hexahydropyridine, triisopropylsilane (TIS), ethanedithiol (EDT), anhydrous ether, trifluoroacetic acid (TFA), N- Methylmorpholine (NMM), N-fluorenylmethoxycarbonyl-6-aminocaproic acid (Fmoc-e-Acp-OH), methanol, Fmoc-cysteine (Fmoc-Cys(Trt)-OH), Fmoc -Aspartic acid (Fmoc-Asp(OtBu)-OH), Fmoc-phenylalanin...
Embodiment 2
[0084] This embodiment provides an imaging probe RGDPGYLGFFC-Cy, which can be represented by formula II, and the preparation method of the probe is the same as that in Example 1.
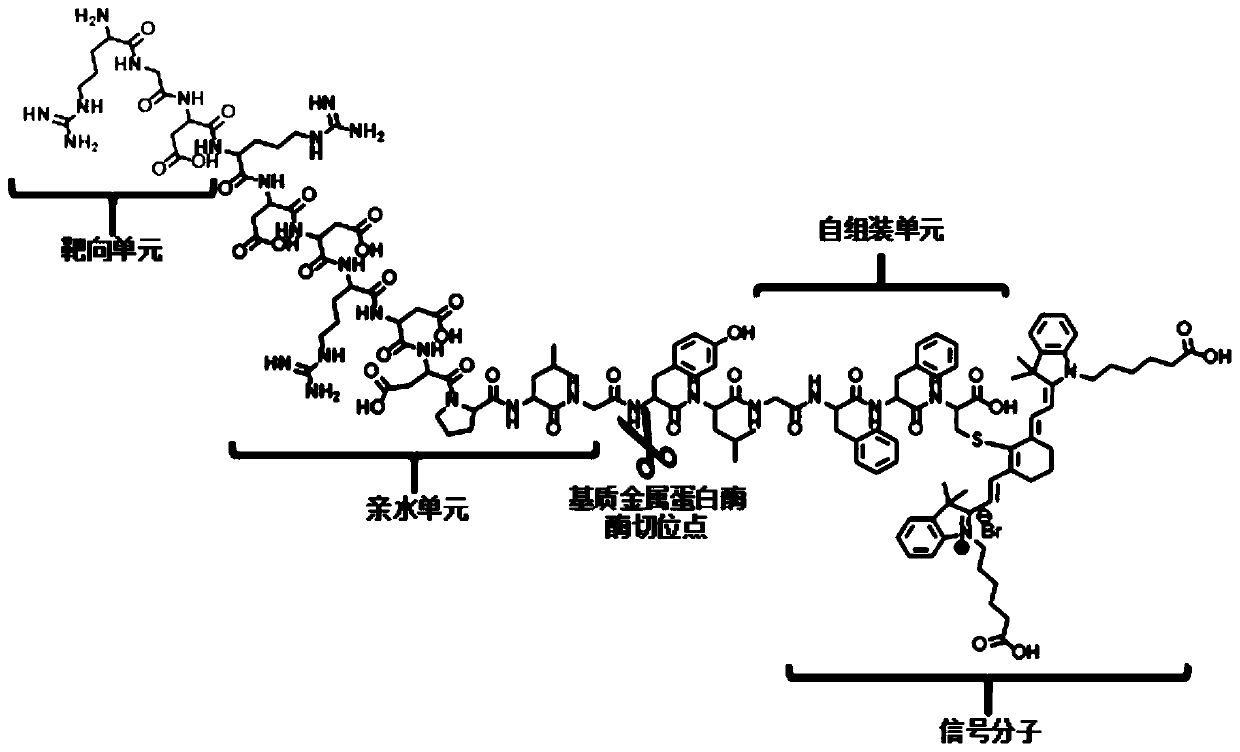
[0085] The polypeptide sequence RGD is the target recognition unit, PLGYLG is the enzyme hydrolysis substrate unit, the enzyme hydrolysis site is located between G and Y, FFC is the self-assembly unit, and C is used to connect the fluorescent group Cy.
Embodiment 3
[0087] This embodiment provides an imaging probe SCYNTNHVPLSPKYGPAKLVFFC-Cy, which can be represented by formula III, and its preparation method is the same as that of Example 1.
[0088] The receptor corresponding to the fragment SCYNTNHVPLSPKY in the polypeptide is carbonic anhydrase-9, the fragment GPA is the hydrolysis substrate unit of fibroblast activation protein-α, the molecular cleavage site is located between P and A, and the polypeptide sequence KLVFFC is a self-assembled fragment. Amino acid C is used to attach the fluorophore.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com