Compositions and methods for treating ocular pathologies
An eye and drug technology, applied in chemical instruments and methods, drug combinations, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1. Treatment of herpetic keratitis
[0179] Serotonin or 5-hydroxytryptamine (5-HT) is a small monoamine molecule known primarily for its role as a neurotransmitter. 5-HT regulates a variety of behaviors in the brain, including cognition, mood, aggression, mating, feeding, and sleep (Nichols and Nichols, 2008). These actions are mediated through seven different receptor families (5-HT 1-7 ), these families contain fourteen distinct subtypes (Nichols and Nichols, 2008). Except 5-HT 3 Receptors are ligand-gated ion channels, each of which is a G protein-coupled receptor. Of all the serotonin receptors, 5-HT is known to couple mainly to the Gαq effector pathway 2A The receptor (Roth et al., 1986) is the one most closely related to complex behavior.
[0180] Agonists of the serotonin receptor pathway, such as DOIs, can be delivered systemically or locally (eg, by topical eye drops) to prevent and abrogate pathogen-selected host-mediated disease processes. Such...
Embodiment 2
[0186] Example 2: Development of preclinical models
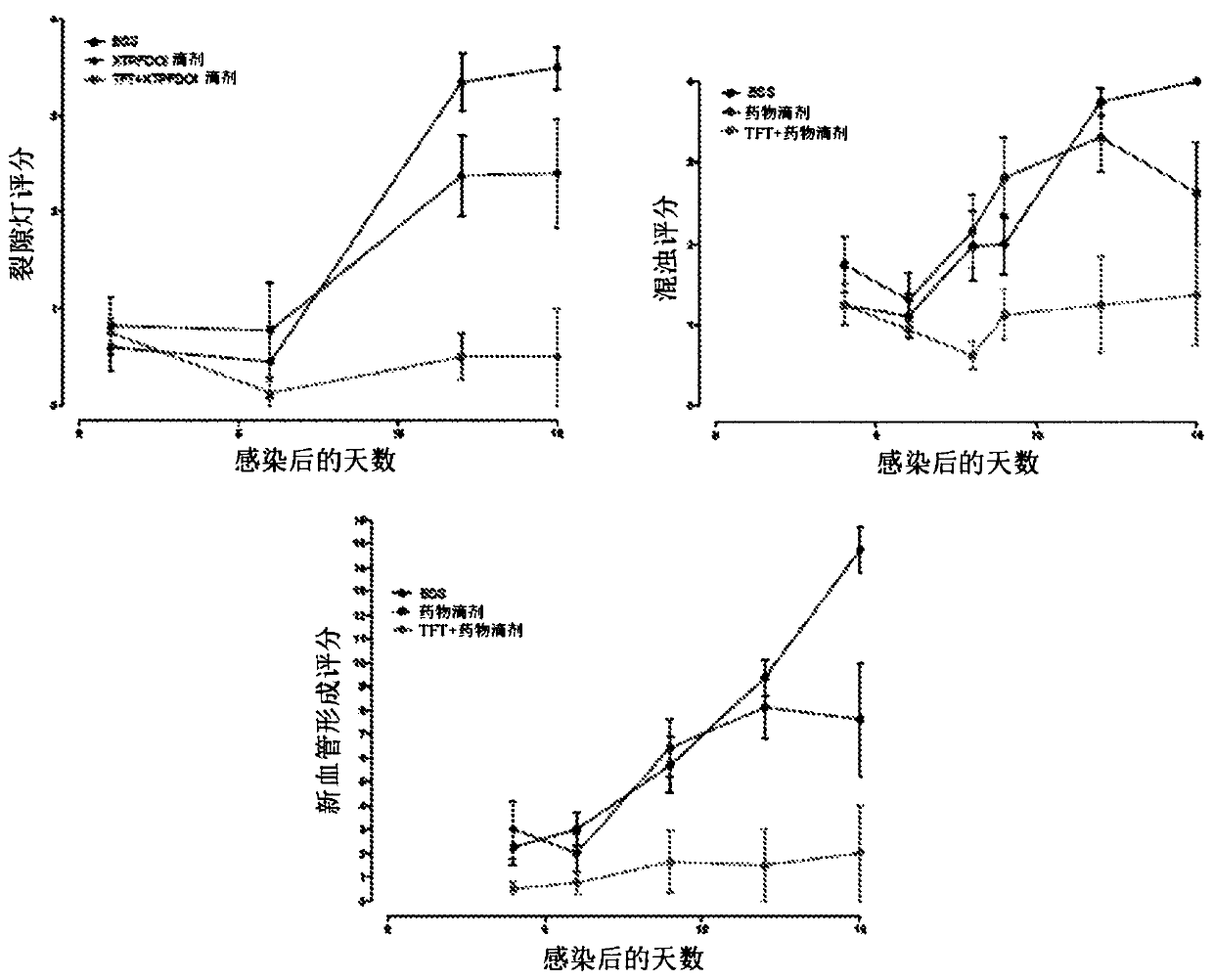
[0187] The data presented above demonstrate that DOI can effectively inhibit disease-associated ocular vascularization, thereby preventing the chronic pathology often associated with disease progression. Without wishing to be bound by theory, DOIs may regulate angiogenesis and vascular homeostasis in these disease processes through direct or indirect effects on vascular cells.
[0188] To assess the effect of DOI on ocular herpetic stromal keratitis associated with pathological angiogenesis, other animal model systems can be developed as described herein. The model system described can be complemented by established in vitro mechanistic studies to assess the direct effects of DOIs on vascular cell biology and function. The contribution of 5-HT receptors in this disease process can likewise be explored.
[0189] Assessing therapeutic efficacy of 5-HT receptor modulation for ameliorating pathological conditions associated w...
Embodiment 3
[0195] Example 3: Formulation Development and Validation
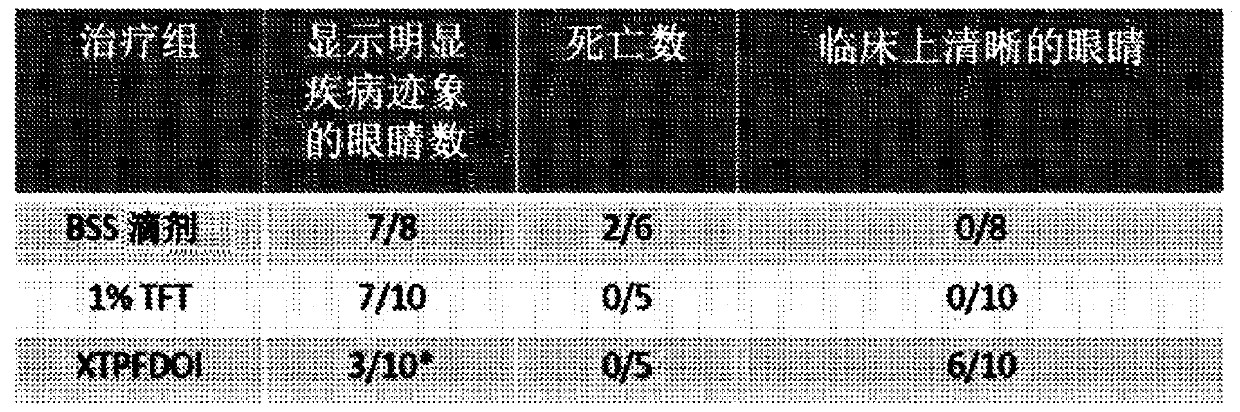
[0196] Aspects of the invention are directed to DOI, a 5-HT that can treat herpetic keratitis 2 R agonist. In the study, (R)-DOI exhibited anti-angiogenic properties in mouse models of primary and chronic herpetic keratitis. Furthermore, (R)-DOI inhibited HSV-1 reactivation from latency in an isolated neuronal model, a major factor in the development of recurrent herpetic stromal keratitis. Without wishing to be bound by theory, the experiments described herein (i) demonstrate that (R)-DOI improves blood vessel formation associated with herpetic keratitis, (ii) develop formulations and methods for effective (R)-DOI ophthalmic delivery. Dosing parameters, and (iii) a CNS safety profile for ophthalmic (R)-DOI was established.
[0197] Tolerability and Pharmacokinetic Parameters of Ophthalmic Preparations of (R)-DOI
[0198] A series of experiments aimed at determining the dosing parameters, including ocular tolerab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com