Process method for producing xylene from C<9><+> heavy aromatic hydrocarbons
A technology for heavy aromatic hydrocarbons and process methods, which is applied in the production of bulk chemicals, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. requirements and other issues, to achieve the effect of low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Vector preparation
[0061] Na 2 O less than 0.05% (weight), SiO 2 / Al 2 o 3 66.7 grams of ammonium ZSM-5 zeolite with a molecular ratio of 80 and Na 2 γ-Al with O less than 0.05% by weight 2 o 3 ·H 2 O 57.1 grams are evenly mixed, then add 7 grams of nitric acid aqueous solution with a concentration of 65-68wt%, 2 grams of scallop powder and 60-70 grams of water, knead evenly, extrude, dry at 120 ° C for 4 hours, and roast at 550 ° C in an air atmosphere After 3 hours, pelletize to obtain a cylindrical carrier with a length of 2 mm and a diameter of 1.5 mm.
[0062] Catalyst preparation
[0063] Get 50 grams of carrier, with 35 milliliters of ammonium molybdate aqueous solution (equivalent to containing MoO 3 1.6 g) was impregnated with the support, dried at 120°C for 4 hours, and calcined at 550°C for 3 hours to obtain MoO 3 Catalyst in an amount of 3.1w%.
[0064] Catalyst evaluation
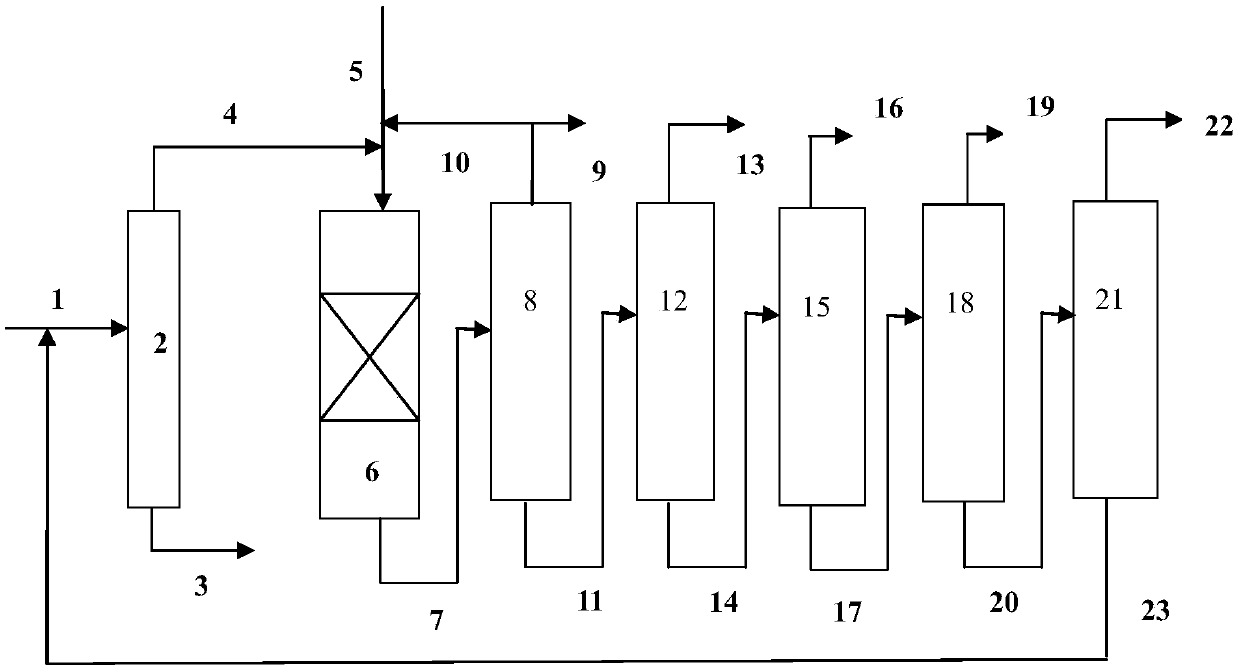
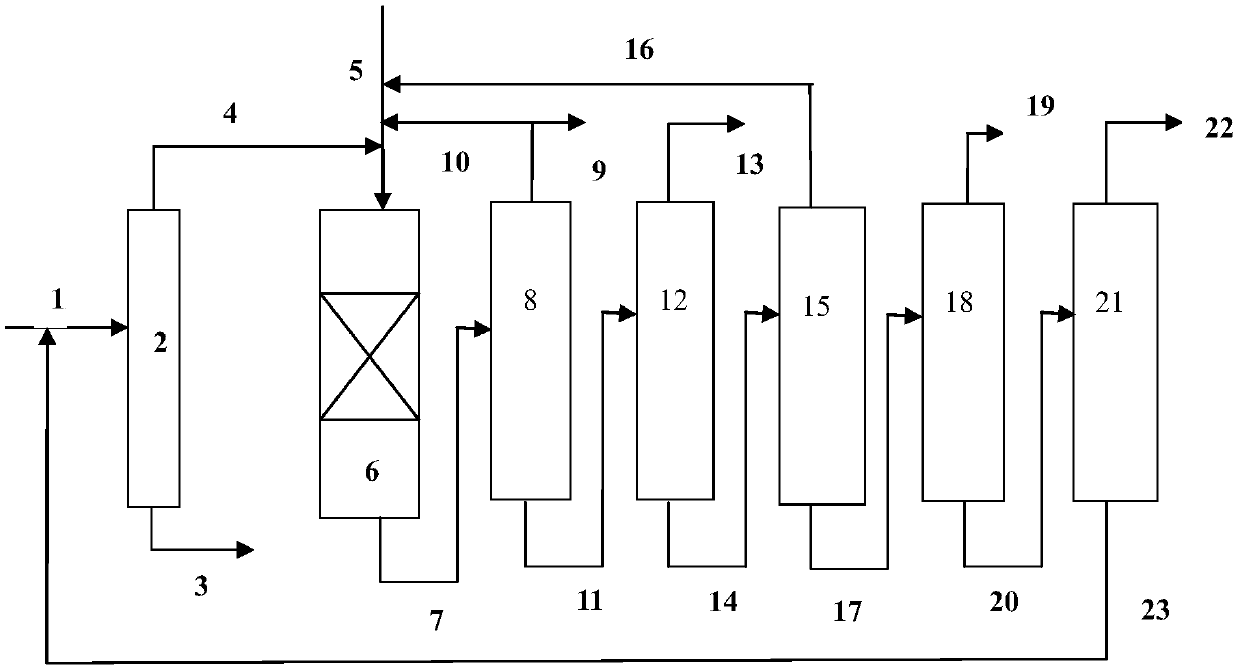
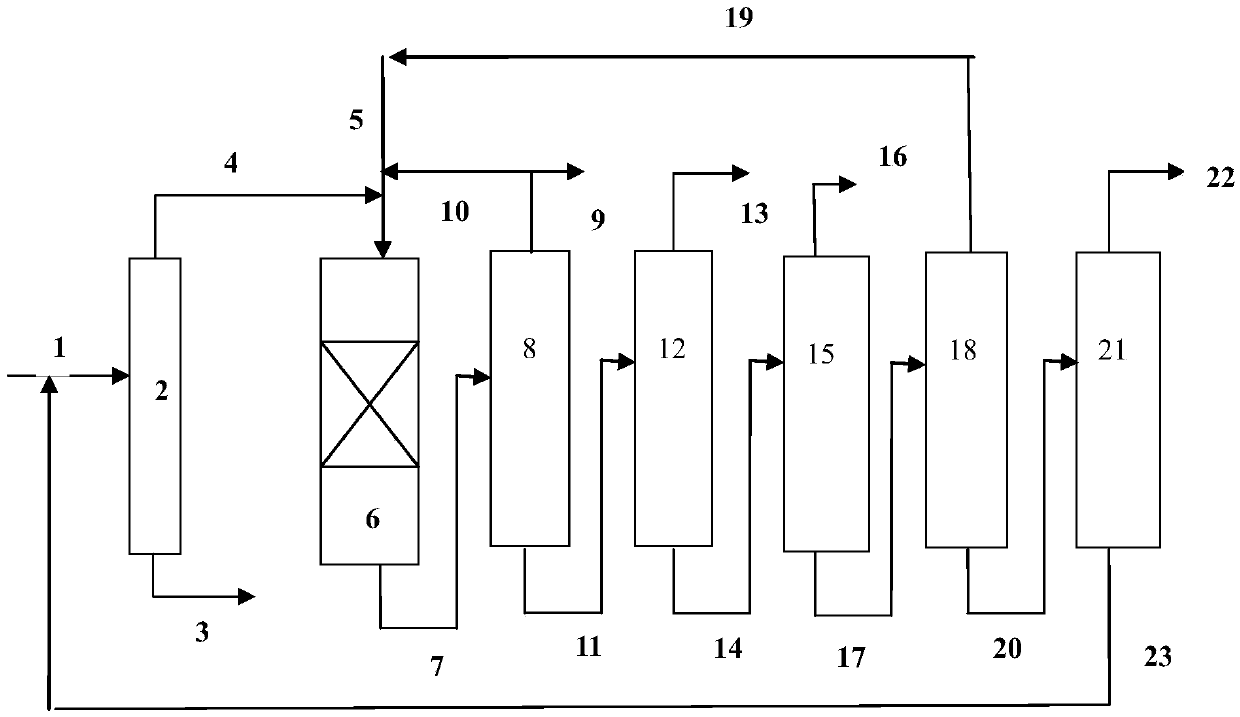
[0065] use figure 1 In the process flow shown, the hydrodealkylation ...
Embodiment 2
[0075] Vector preparation
[0076] Na 2 O less than 0.05% (weight), SiO 2 / Al 2 o 3 66.7 grams of ammonium ZSM-5 zeolite with a molecular ratio of 80 and Na 2 γ-Al with O less than 0.05% by weight 2 o 3 ·H 2 O 57.1 grams are evenly mixed, then add 7 grams of nitric acid aqueous solution with a concentration of 65-68wt%, 2 grams of scallop powder and 60-70 grams of water, knead evenly, extrude, dry at 120 ° C for 4 hours, and roast at 550 ° C in an air atmosphere After 3 hours, pelletize to obtain a cylindrical carrier with a length of 2 mm and a diameter of 1.5 mm.
[0077] Catalyst preparation
[0078] Get 50 grams of carrier, with 35 milliliters of ammonium molybdate aqueous solution (equivalent to containing MoO 3 1.6 g) was impregnated with the support, dried at 120°C for 4 hours, and calcined at 550°C for 3 hours to obtain MoO 3 Catalyst in an amount of 3.1w%.
[0079] Catalyst evaluation
[0080] use figure 1 In the process flow shown, the hydrodealkylatio...
Embodiment 3
[0090] Vector preparation
[0091] Na 2 O less than 0.05% (weight), SiO 2 / Al 2 o 3 66.7 grams of ammonium Beta zeolite with a molecular ratio of 25 and Na 2 γ-Al with O less than 0.05% by weight 2 o 3 ·H 2 O 57.1 grams are evenly mixed, then add 7 grams of nitric acid aqueous solution with a concentration of 65-68wt%, 2 grams of scallop powder and 60-70 grams of water, knead evenly, extrude, dry at 120 ° C for 4 hours, and roast at 550 ° C in an air atmosphere After 3 hours, pelletize to obtain a cylindrical carrier with a length of 2 mm and a diameter of 1.5 mm.
[0092] Catalyst preparation
[0093] Take 50 grams of carrier, impregnate the carrier with 35 milliliters of nickel nitrate aqueous solution (equivalent to 1.3 grams of NiO), dry at 120°C for 4 hours, and roast at 550°C for 3 hours to prepare a catalyst with a NiO content of 2.5w%.
[0094] Catalyst evaluation
[0095] use figure 1 In the process flow shown, the hydrodealkylation and transalkylation rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com