Anti CD70 CAR-T cell and preparation method and application thereof
A cell and lymphocyte technology, applied in the fields of molecular biology and cell biology, can solve problems such as good curative effect, achieve high-efficiency tumor killing activity, and increase the specificity of T cell killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
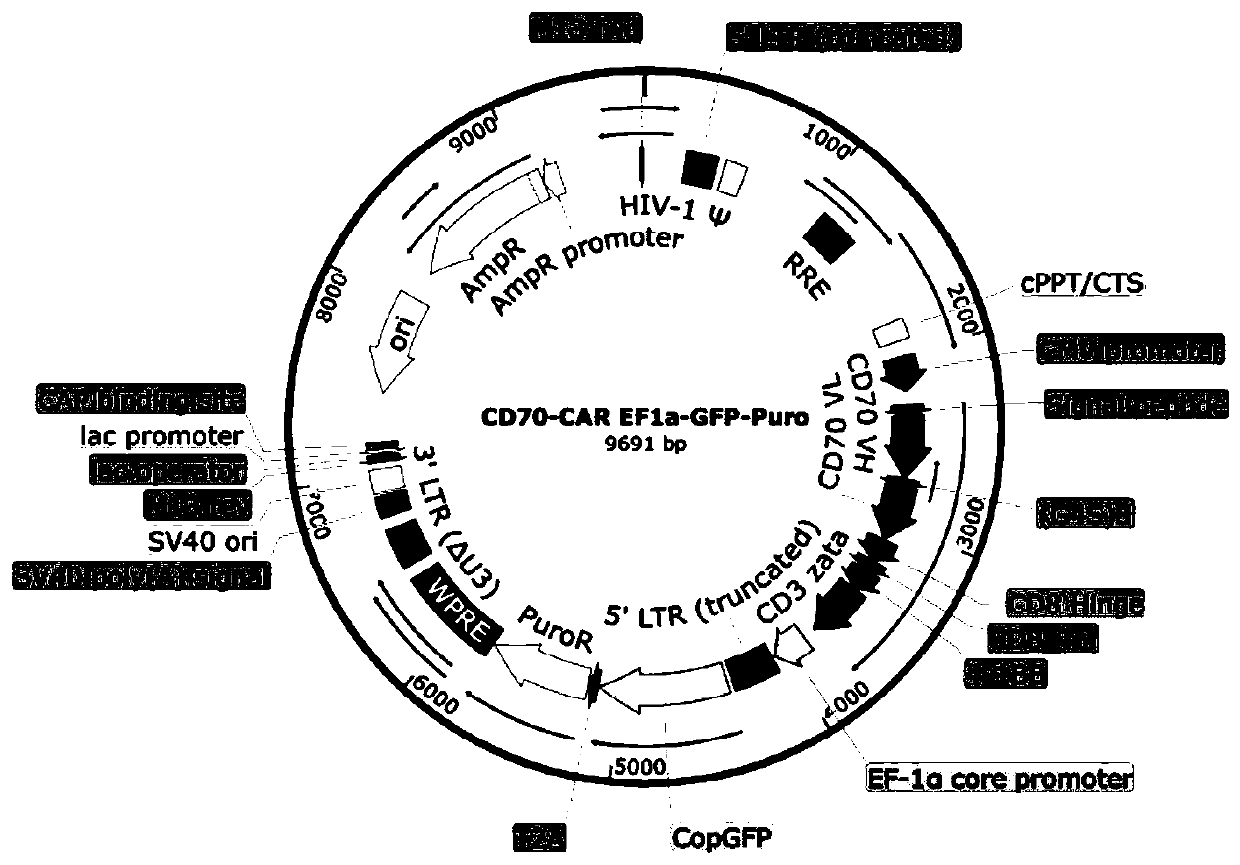
[0044] Example 1: Preparation of lentiviral expression vector
[0045] Gene synthesis of SCFV(anti CD70)-CD8 TM -4-1BB-CD3ζ fusion gene sequence, the gene sequence is shown in SEQ ID NO.7, which is connected to the PLV vector through enzyme digestion transformation, and the upstream of the gene is the EP-1a promoter. Transform the DH5α Escherichia coli strain with the vector, screen with ampicillin, obtain positive clones, extract plasmids, identify the clones by enzyme digestion, and obtain PLV-SCFV(antiCD70)-CD8 TM -4-1BB-CD3ζ lentiviral packaging vector, see figure 1 .
Embodiment 2
[0046] Example 2: Preparation of lentivirus
[0047] 24 hours before transfection, use about 8×10 per bottle 6 293T cells were inoculated into T75 culture flasks. Make sure that the cells are about 80% confluent and evenly distributed in the culture flask for lentiviral packaging.
[0048] Prepare Plasmid and Transfection Reagent Dilutions
[0049] 1. Vortex to mix the PEI 40K transfection reagent.
[0050] 2. Prepare 2 centrifuge tubes, and prepare plasmid and transfection reagent dilutions in the following order.
[0051]
[0052] 3. Mix well.
[0053] 4. Add the transfection reagent dilution (centrifuge tube 2) into the plasmid DNA solution (centrifuge tube 1), and mix thoroughly immediately. Note that the join order is very important.
[0054] 5. Incubate the transfection mixture at room temperature for 15-20 minutes.
[0055] 6. Add 1ml of the transfection mixture into the well-prepared 293T cell culture flask, and gently blow and aspirate the medium to mix.
...
Embodiment 3
[0059] Example 3: Preparation of Anti CD70 CAR-T cells
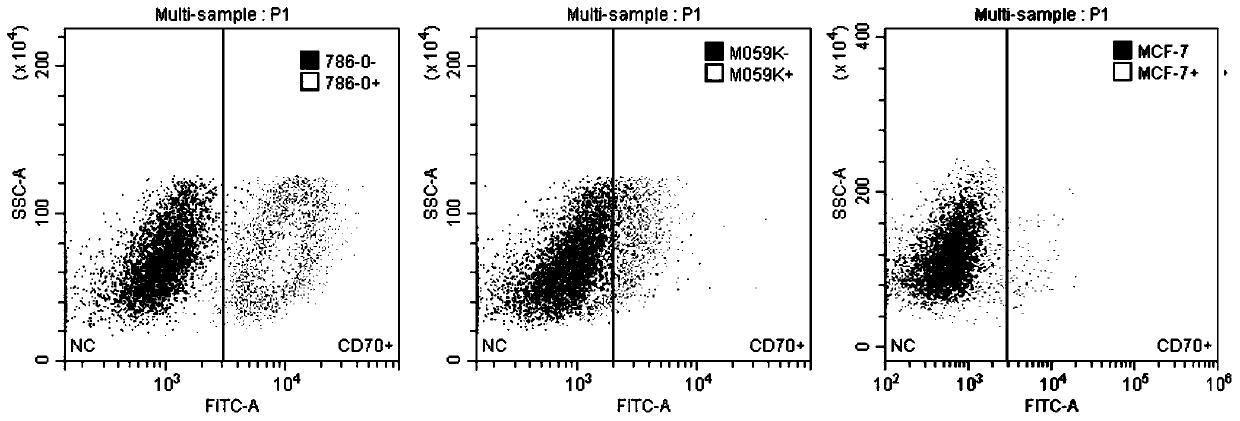
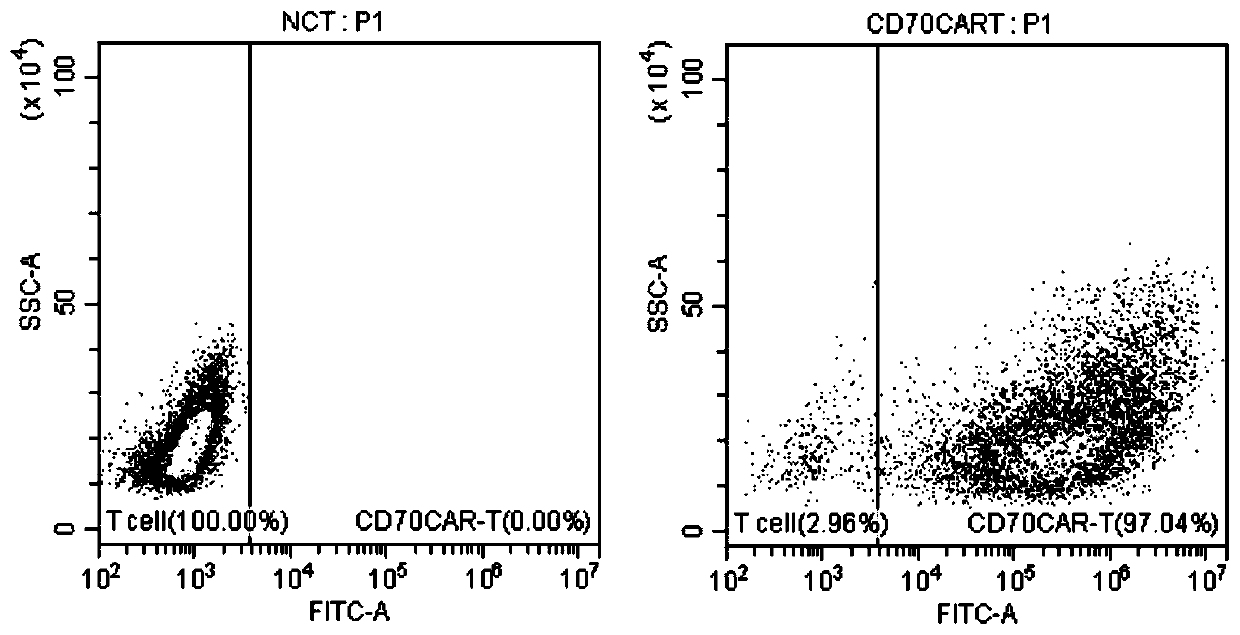
[0060] Take 0.5ml of blood for rapid detection of pathogenic microorganisms to rule out microbial infections such as HBV, HCV, HDV, HEV, HIV-1 / 2, Treponema pallidum and parasites. Peripheral blood mononuclear cells (PBMC) were collected from the patients by an apheresis machine. Configure complete growth medium, add 5% autologous AB or FBS to PBSPBS, IL-2 concentration is 20ng / ml, dilute the isolated PBMC with medium to 2×10 / ml, take 50u1 flow cytometric detection of T cells in PBMC purity. On day 0, configure buffer1, add 1% FBS to PBS, shake the beads for 30s or manually shake up and down for 5min, take out CD3 / CD8beads according to the ratio of beads to T cells 3 to 1, put them in a 1.5ml EP tube, add 1ml Buffer1 Wash the beads, then use a magnet to suck the beadslmin from the EP tube, discard the washing solution, repeat twice, then use the medium to resuspend the beads to the original volume, mix the cells and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com