Pair of quinazolinone alkaloid enantiomers, and preparation method and application thereof
A quinazolinone and the technology of the quinazolinone, which are applied in the field of quinazolinone alkaloid enantiomers and their preparation, can solve problems such as toxic and side effects, and achieve the effect of good anti-inflammatory drug potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Isolation of ascidian symbiotic fungus Penicillium canescens SYSU-MS4829
[0038] 1. Strain isolation:
[0039] Sample: Styela plicata from Tai'ao Bay, Shenzhen.
[0040] Isolation method: Disinfect the surface of fresh Ascidian ascidian, dry it for a while, grind it, inoculate it into PDA medium, Martin medium or Chase medium under aseptic conditions, and culture it below 28°C for 5-7 days to obtain a single strain of Penicillium canescens SYSU-MS4829; the obtained bacterial strain Penicillium canescens SYSU-MS4829 was preserved at 4° C. on a common PDA medium slant.
Embodiment 2
[0041] Example 2 Identification of ascidian symbiotic fungus Penicillium canescens SYSU-MS4829
[0042] 1. Morphological and physiological and biochemical identification:
[0043] The biological characteristics of the strain, when cultured at a constant temperature of 28°C on PDA medium, the surface of the colony is white hairy hyphae, the back is dark red, and green spores are formed.
[0044] 2. Molecular identification:
[0045] The purely cultured DNA of Penicillium canescens SYSU-MS4829 was extracted by the CTAB method, and the ITS-rRNA gene fragment was amplified by a PCR amplification instrument using a pair of primers ITS1F and ITS4 in the ITS spacer region. The reaction system was 50uL. The conditions were: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 40 s, annealing at 52°C for 40 s, extension at 72°C for 1 min, repeating denaturation, annealing and extension for 30 cycles, and finally extension at 72°C for 10 min.
[0046] It was determined by Sephad...
Embodiment 3
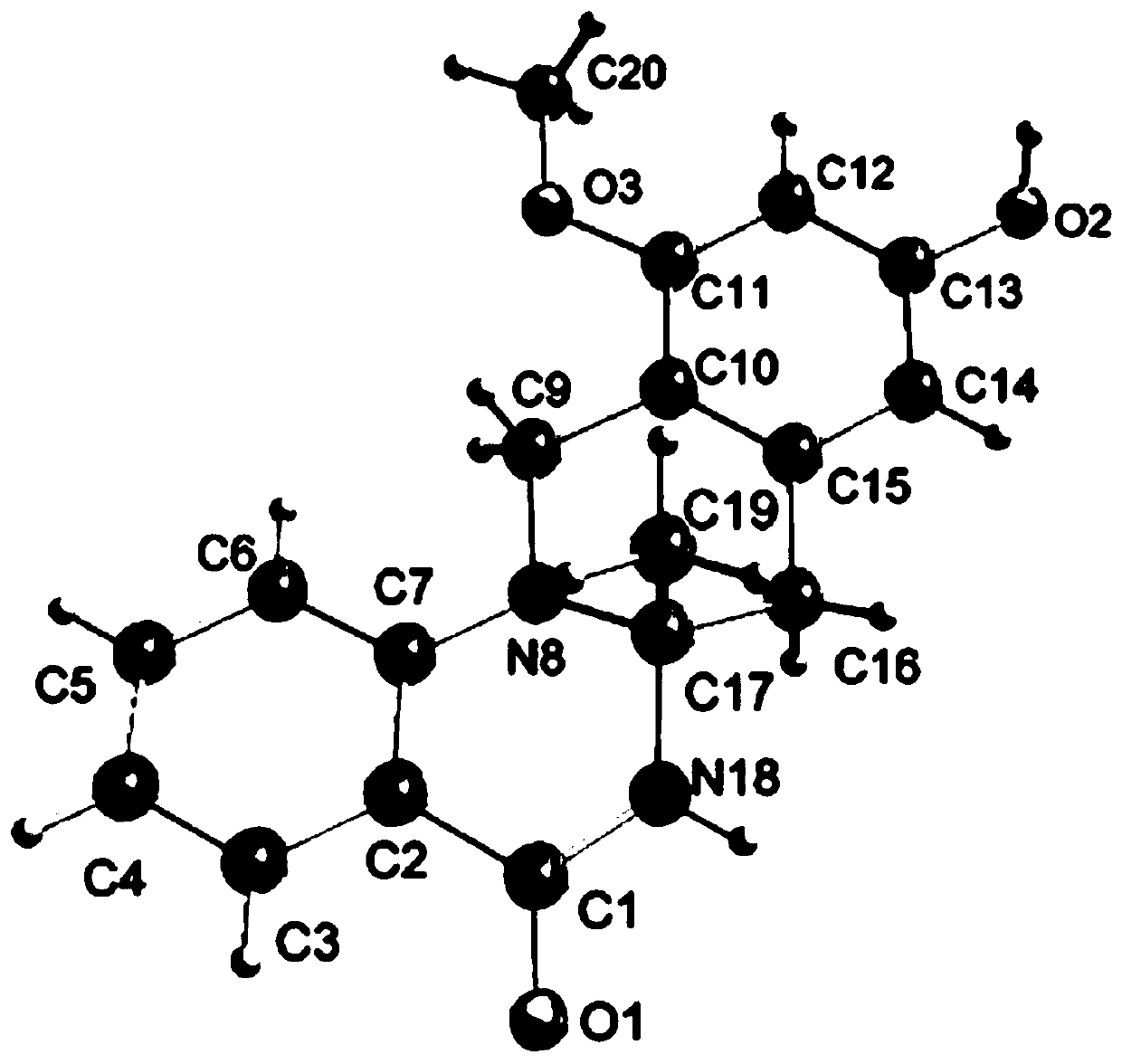
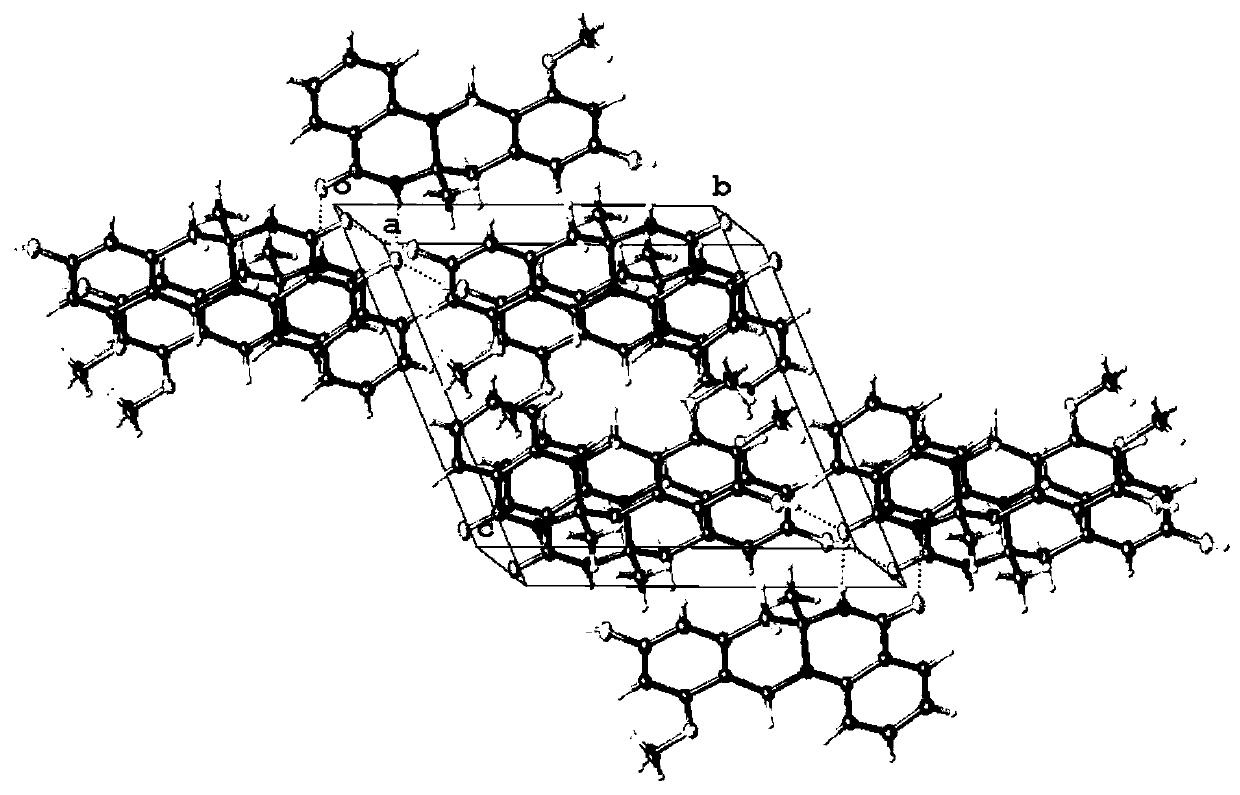
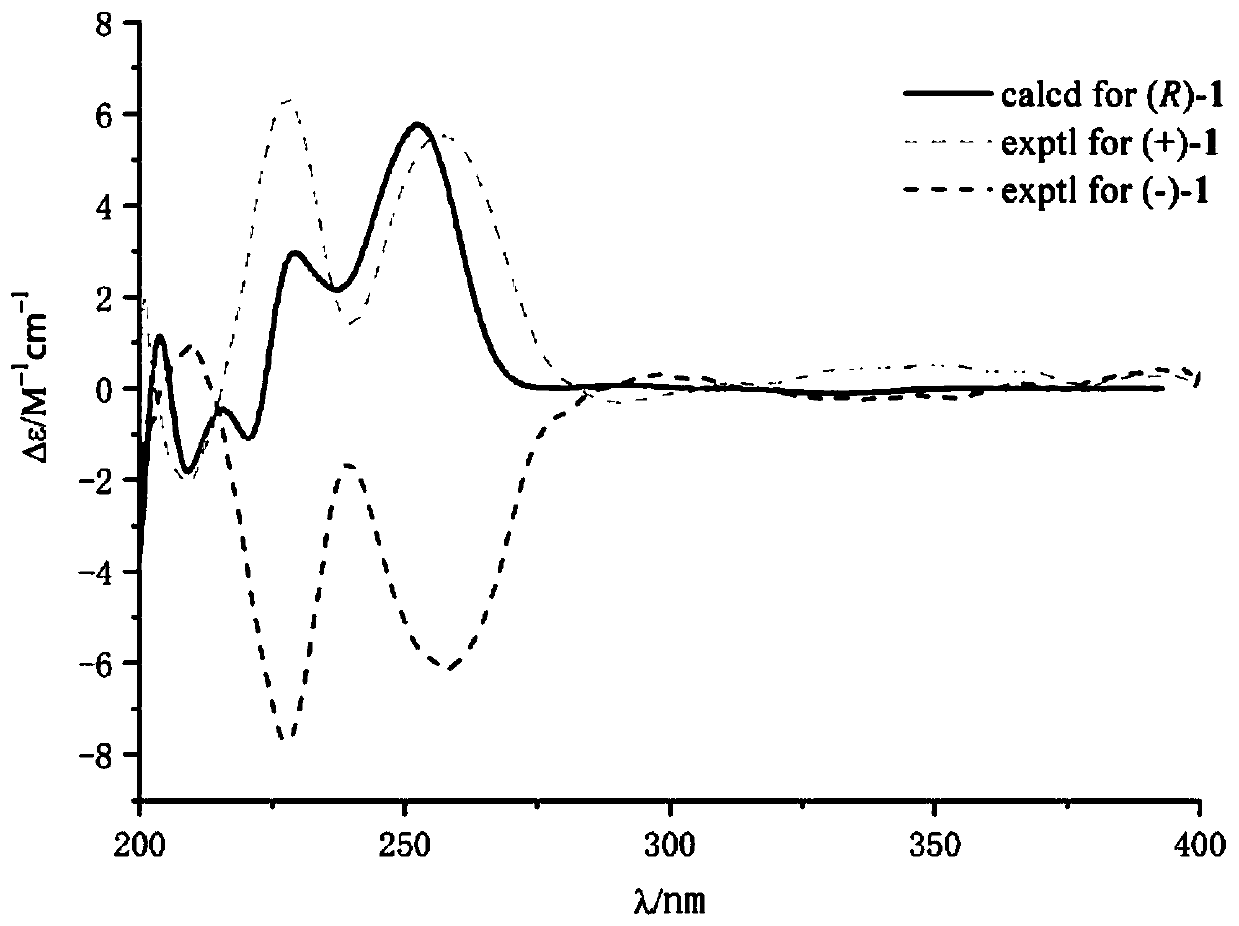
[0048] Example 3 The separation and identification of quinazolinone alkaloid enantiomer (±)-penicamide A
[0049] 1. The separation of quinazolinone alkaloid enantiomer (±)-penicamide A, the specific process is as follows:
[0050] S1. The enlarged culture of the symbiotic fungus Penicillium canescens SYSU-MS4829, and obtain the thalline of the symbiotic fungus ascidian;
[0051] The specific expansion process is as follows:
[0052] S11. Preparation of seed medium: 200g of potatoes, 20g of glucose, 1L of tap water, prepared as a medium according to a conventional method, evenly distributed in five 500mL Erlenmeyer flasks, and extinguished at 121°C for 30 minutes;
[0053] S12. Cultivation of seeds: Inoculate the strain of the marine fungus Penicillium canescens SYSU-MS4829 into the seed medium, place it on a shaker at a speed of 180 rpm at a temperature of 28°C, and cultivate it for 120 hours to obtain a seed culture solution;
[0054] S13. Preparation of fermentation mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com