Fluorescence sensing material, preparation method thereof and application in high-sensitivity distinguished detection of chemical warfare agents
A technology of fluorescent materials and chemical warfare agents, applied in the field of organic fluorescent sensing materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
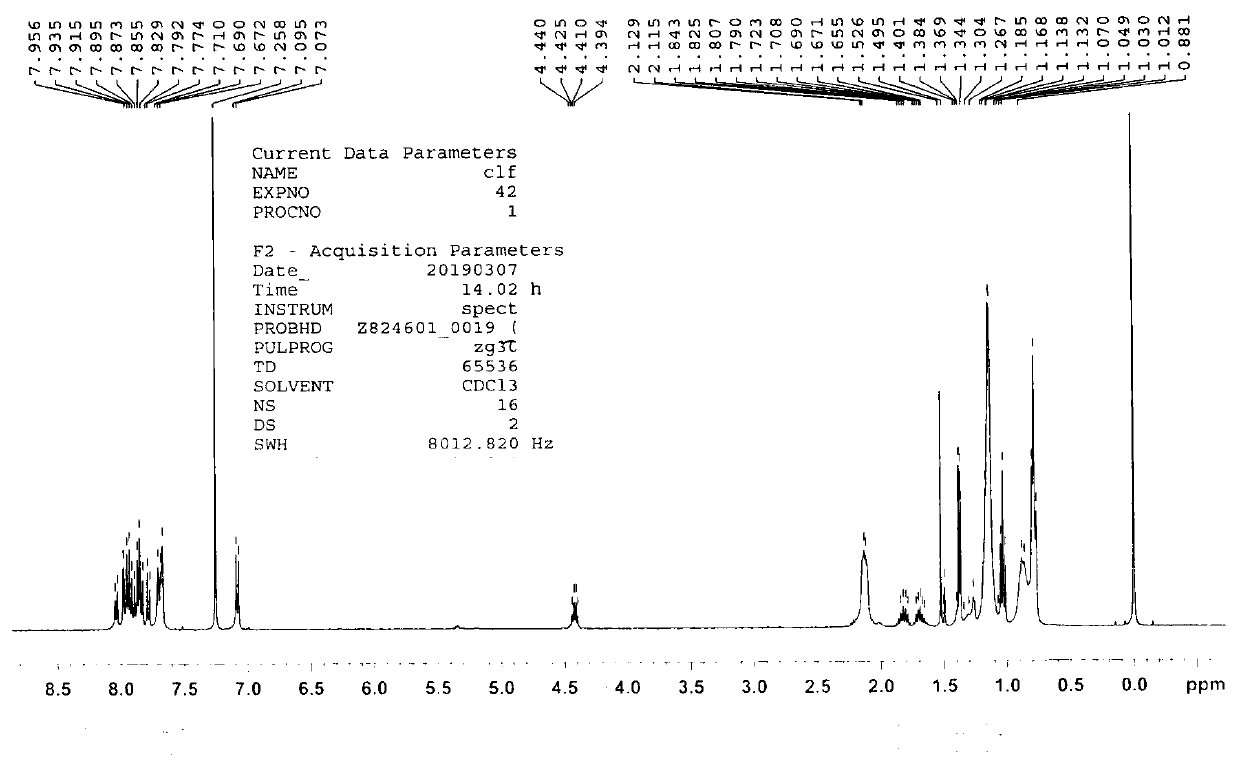
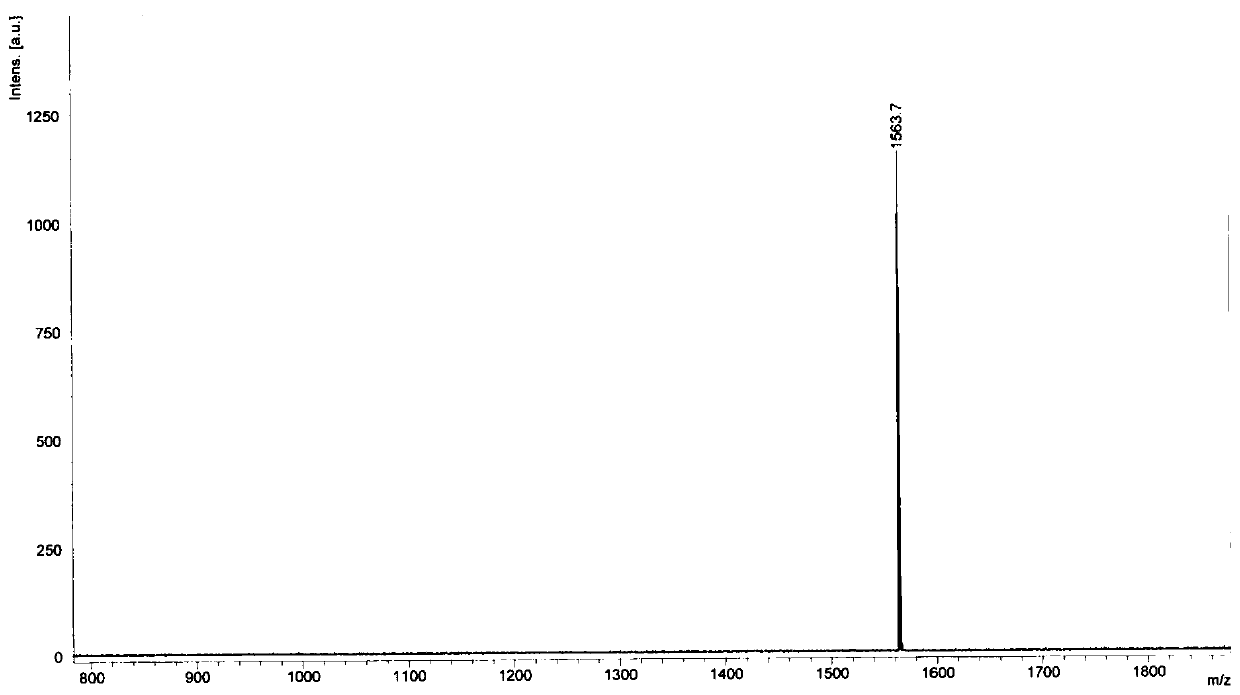
[0137] Prepare molecule A with the formula 1 .
[0138]
[0139] (1) Add 3.0 grams of 4-bromophenol, 1.5 grams of (S)-2-butanol, 5.4 grams of triphenylphosphine and 40 milliliters of tetrahydrofuran into a 100ml round-bottomed flask. Slowly add 4.2 g of diisopropyl azodicarboxylate, rise to room temperature, stir for 5 hours, and separate by column chromatography to obtain 1-bromo-4-((R)-sec-butoxy)benzene;
[0140] (2) Add 3.0 grams of the product obtained in step (1) to a 100ml round bottom flask, add 4.0 grams of pinacol ester boronate, 3.8 grams of potassium acetate and 0.5 grams of 1,1'-bis(diphenylphosphine base) ferrocene palladium dichloride (II), 30 milliliters of 1,4-dioxane, reacted overnight at 80°C under Ar protection, and obtained 2-(4-((R)-sec-butyl Oxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
[0141] (3) Add 1.5 grams of the product obtained in step (2) to a 100ml round bottom flask, 1.9 grams of 4,7-dibromobenzothiadiazole, 0.3 gram of tetraphen...
Embodiment 2
[0154] Prepare molecule B with the formula 1 .
[0155]
[0156] (1) Add 0.86 gram of 4-(4-pyridyl) phenylboronic acid, 1.5 gram of 4,7-dibromobenzothiadiazole, 0.25 gram of tetraphenylphosphine palladium, 1.78 gram of potassium carbonate in a 100ml round bottom flask , 30 milliliters of 1,4-dioxane and 6 milliliters of water were reacted overnight at 80°C under the protection of Ar, and 4-bromo-7-(4-(4-pyridyl)phenyl)benzo was obtained after separation by column chromatography Thiadiazole;
[0157] (2) Add 1.2 grams of the product obtained in step (1) to a 100ml round bottom flask, add 0.99 grams of pinacol ester boronate, 0.96 grams of potassium acetate and 0.12 grams of 1,1'-bis(diphenylphosphine Base) ferrocene palladium dichloride (II), 30 milliliters 1,4-dioxane, 80 ℃ overnight reaction under Ar protection, obtain 2-(4-(4-pyridyl) benzene after column chromatography separation base)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
[0158] (3) 1.3 grams of products obtain...
Embodiment 3
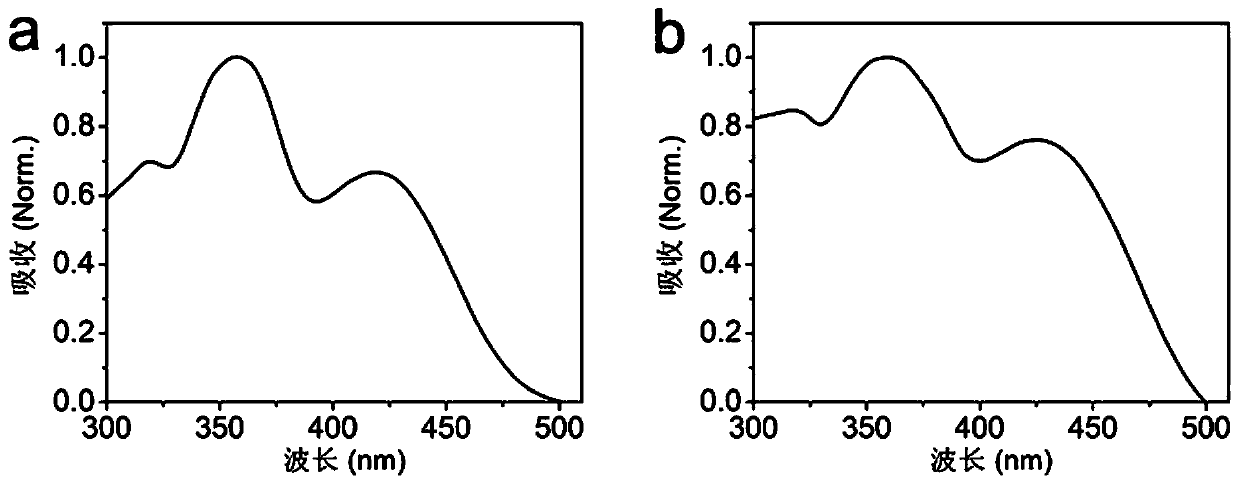
[0169] By the molecular structure A prepared by embodiment 1 1 A film composed of self-assembled nanoparticles was used for the fluorescence detection of mustard gas and sarin.
[0170] will be given by molecular structure A 1 The self-assembled nanoparticle aggregates are coated in self-made quartz tubes, and then loaded into the detection equipment independently developed by the laboratory. The microsphere aggregates are excited by a 385 nm excitation light source, and the light intensity at 520-560 nm is integrated. , plotting light intensity versus time. The vapors of mustard gas or sarin of different concentrations were sucked with a 10 ml syringe, and blown into the quartz tube at a speed of 1 ml / s. The test results showed that the blowing of mustard gas of different concentrations all led to fluorescence quenching (such as Figure 11 shown), sarin insufflation does not cause fluorescence quenching (eg Figure 12 shown). Based on this, we propose material A1 The mech...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com