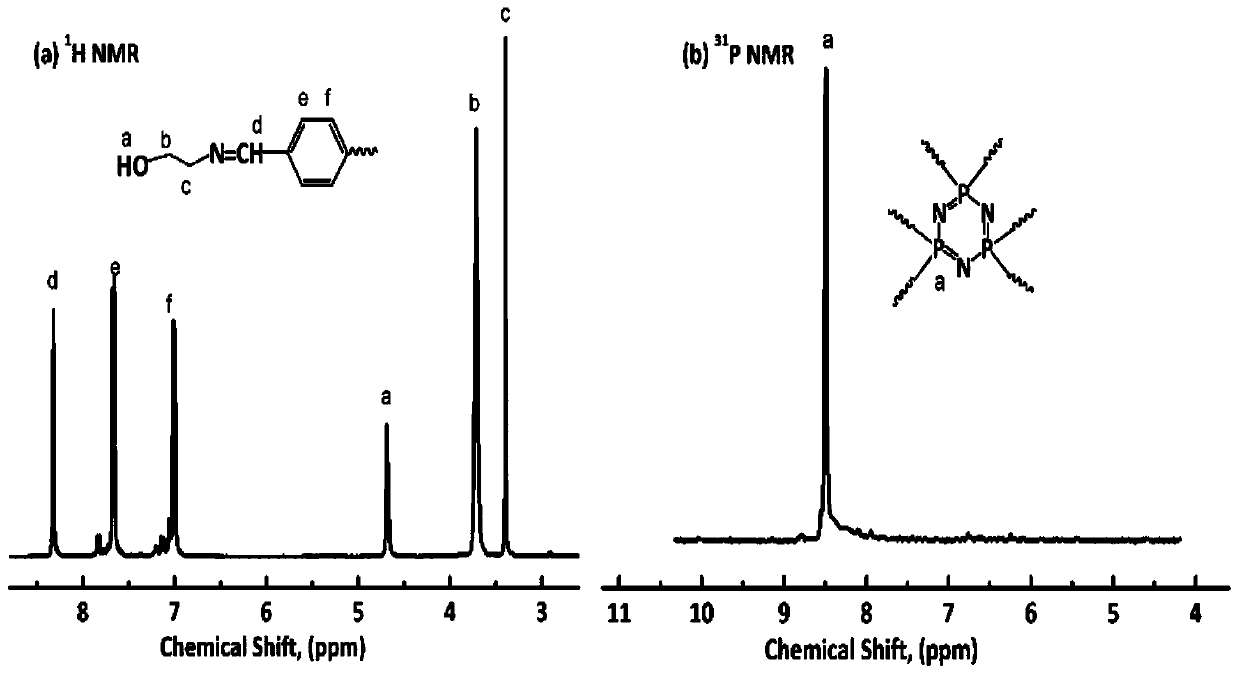
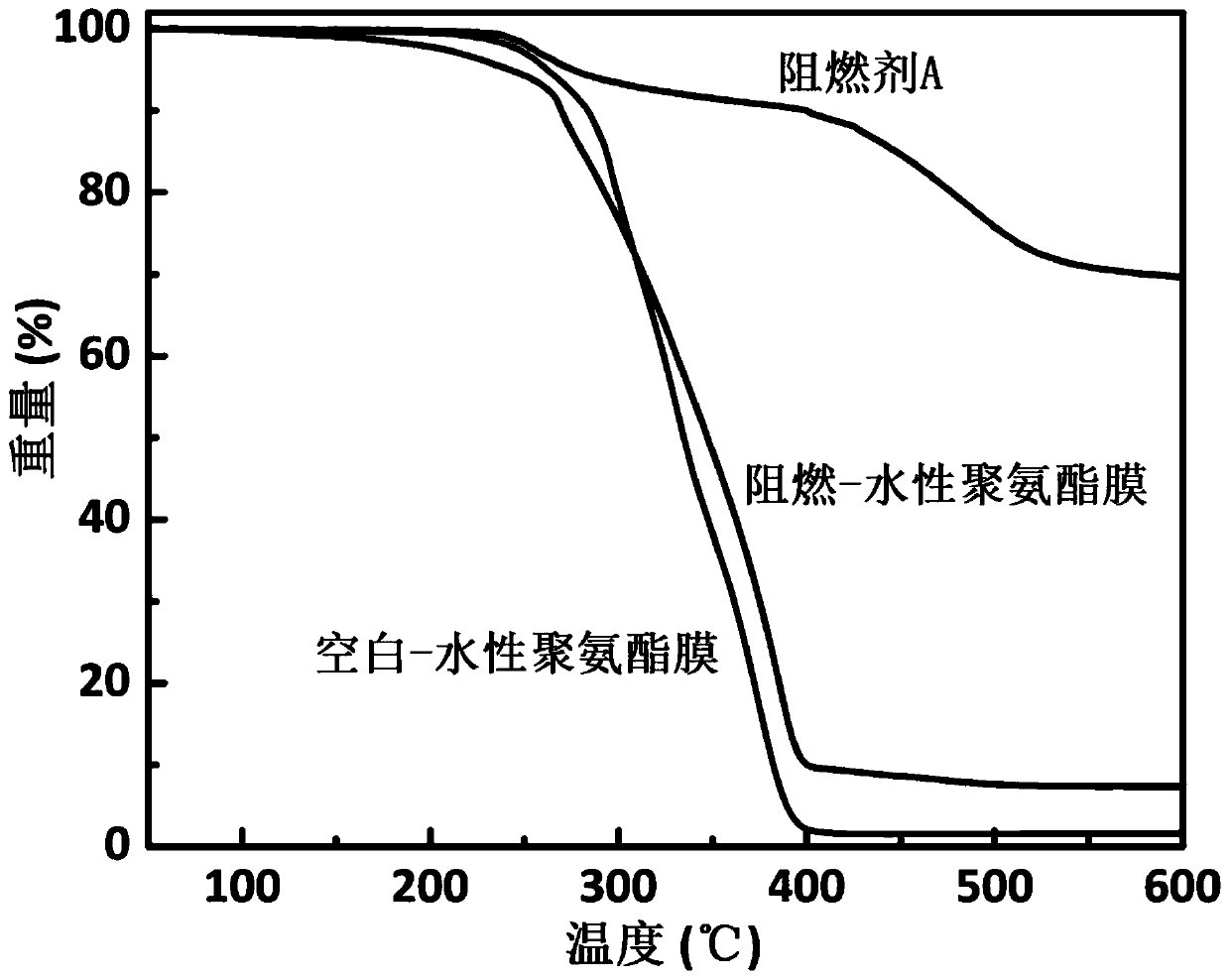
Synthesis methods of Schiff base phosphazene double-base structure flame retardant and modified polyurethane flame retardant
A polyurethane flame retardant, Schiff base phosphazene technology, applied in the field of flame retardants, can solve the problems of secondary combustion, high temperature burns, etc., achieve the effects of small addition, stable flame retardant performance, and improved mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A method for synthesizing a Schiff base phosphazene double-base structure flame retardant, comprising the steps of:
[0034] S1. Add 0.16mol p-hydroxybenzaldehyde, 0.25mol potassium carbonate and 200ml tetrahydrofuran into a four-necked round-bottomed flask equipped with a mechanical stirrer, a condenser, a constant pressure funnel and a nitrogen protection device, and pass into the round-bottomed flask High-purity nitrogen, until the flask reaches anaerobic and water-free conditions, start stirring until the reaction system is clear and transparent; dissolve 0.024mol hexachlorocyclotriphosphazene in 100ml tetrahydrofuran, drop it into the reaction vessel through a constant pressure funnel, and then The reaction was stirred and reacted at 65°C for 24 hours, and the crude product obtained from the reaction was subjected to rotary evaporation, water washing, recrystallization and drying to obtain white crystals, which were intermediate products, with a yield of 93.1%;
[...
Embodiment 2
[0056] A method for synthesizing a Schiff base-containing phosphazene double-radical structure flame retardant comprises the following steps:
[0057] S1. Add 0.16mol 2-hydroxypyridine-5-aldehyde, 0.25mol potassium carbonate and 200ml tetrahydrofuran into a four-necked round-bottomed flask equipped with a mechanical stirrer, a condenser tube, a constant pressure funnel and a nitrogen protection device. Introduce high-purity nitrogen into the flask until the anaerobic and water-free conditions are reached in the flask, and start stirring until the reaction system is clear and transparent; dissolve 0.024mol hexachlorocyclotriphosphazene in 100ml tetrahydrofuran, and drop it into the reaction vessel through a constant pressure funnel , then stirred and reacted at 65°C for 24h, and the crude product obtained in the reaction was subjected to rotary evaporation, water washing, recrystallization and drying to obtain a phosphazene-containing intermediate;
[0058] S2. Add 0.02mol of t...
Embodiment 3
[0062] A method for synthesizing a Schiff base-containing phosphazene double-radical structure flame retardant comprises the following steps:
[0063] S1. Add 0.16mol 5-(4-hydroxypiperidine)-2-thiophenecarbaldehyde, 0.25mol potassium carbonate and 200ml tetrahydrofuran into a four-hole round bottom equipped with a mechanical stirrer, condenser tube, constant pressure funnel and nitrogen protection device In the flask, pass high-purity nitrogen gas into the round-bottomed flask until the flask reaches anaerobic and water-free conditions, start stirring until the reaction system is clear and transparent; dissolve 0.024mol hexachlorocyclotriphosphazene in 100ml tetrahydrofuran, and pass Add the funnel dropwise into the reaction vessel, then stir and react at 65°C for 24 hours, and the crude product obtained from the reaction is rotary evaporated, washed with water, recrystallized and dried to obtain the phosphazene-containing intermediate;
[0064] S2. Add 0.02mol of the phosphaz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com