Pharmaceutically acceptable salt, crystal form and preparation method of pyrazoloheteroaryl derivatives
A technology for medicinal salts and crystal forms, applied in the field of medicinal salts of pyrazoloheteroaryl derivatives, can solve the problems of poor product stability, difficult filtration, easy caking and the like, and achieves high chemical stability, Stable production process and little change in purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
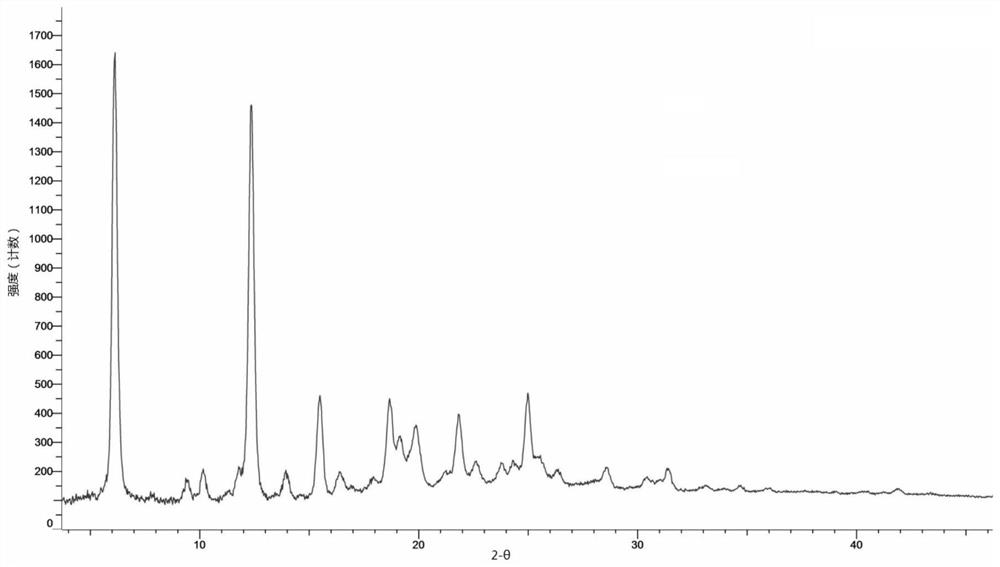
[0132] Weigh 10 mg of the compound shown in formula (I) and add it to the reaction flask, add 0.25 ml of acetonitrile, heat up to 50 ° C, add 3.05 mg of fumaric acid, keep stirring for 30 min, collect the reaction product, and dry it in vacuum at 40 ° C to obtain the formula (I ) A crystal form of compound fumarate shown in ). Its X-ray diffraction pattern is shown in figure 1 , see the TGA spectrum Figure 13 , and its characteristic peak positions are shown in the table below:
[0133] Table 1. Characteristic peaks of crystal form A of fumarate
[0134]
Embodiment 2
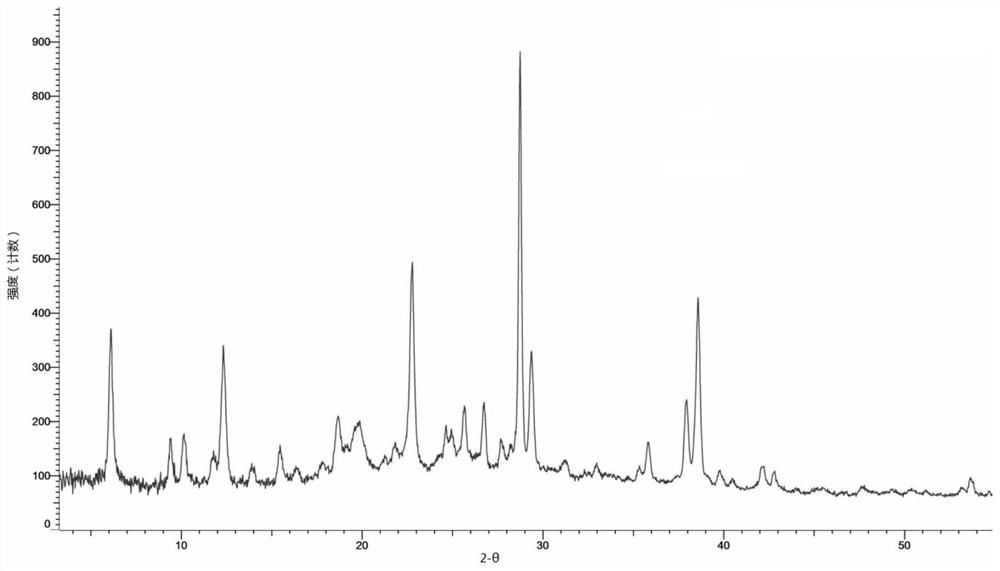
[0136] Weigh 10 mg of the compound represented by formula (I) and add it to the reaction flask, add 0.25 ml of ethyl acetate, heat up to 50 ° C, add 3.05 mg of fumaric acid, keep stirring for 30 min, collect the reaction product, and dry it in vacuum at 40 ° C to obtain the formula (1) The B crystal form of the compound fumarate shown in (I). Its X-ray diffraction pattern is shown in figure 2 , see the TGA spectrum Figure 14 , and its characteristic peak positions are shown in the table below:
[0137] Table 2. Characteristic peaks of fumarate B crystal form
[0138]
Embodiment 3
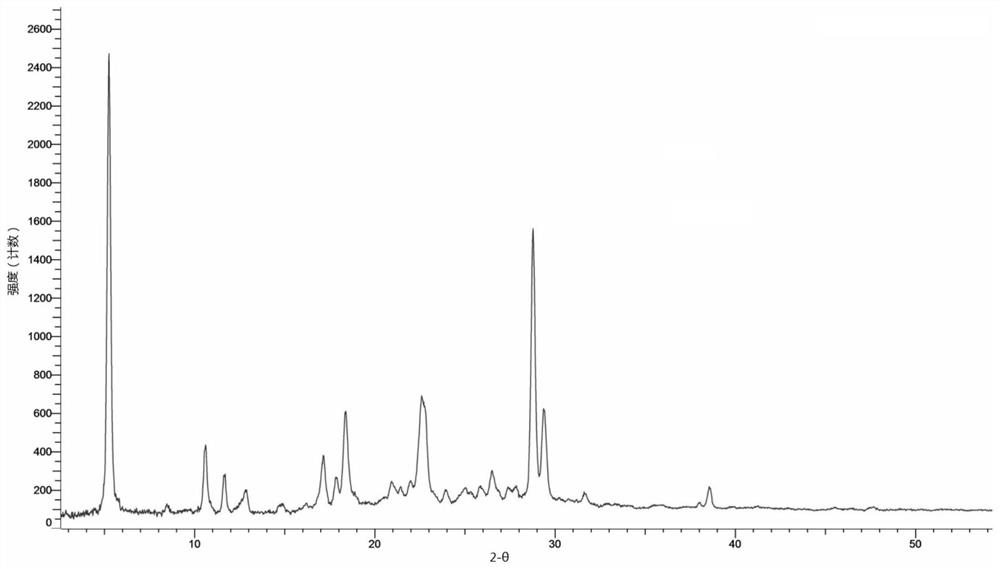
[0140] Weigh 10 mg of the compound shown in formula (I) and add it to the reaction flask, add 0.25 ml of p-xylene, heat up to 50 ° C, add 3.05 mg of fumaric acid, keep stirring for 30 min, collect the reaction product, and dry it in vacuum at 40 ° C to obtain the formula (1) The C crystal form of the shown compound fumarate. Its X-ray diffraction pattern is shown in image 3 , and its characteristic peak positions are shown in the table below:
[0141] Table 3. Characteristic peaks of crystal form C of fumarate
[0142]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com