A kind of phthalocyanine-carboxymethyl chitosan conjugate and its preparation method and application
A technology of carboxymethyl chitosan coupling and carboxymethyl chitosan is applied in the field of phthalocyanine-carboxymethyl chitosan conjugate and its preparation, which can solve few problems and achieve low price and heavy weight. Highly visible, source-rich effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
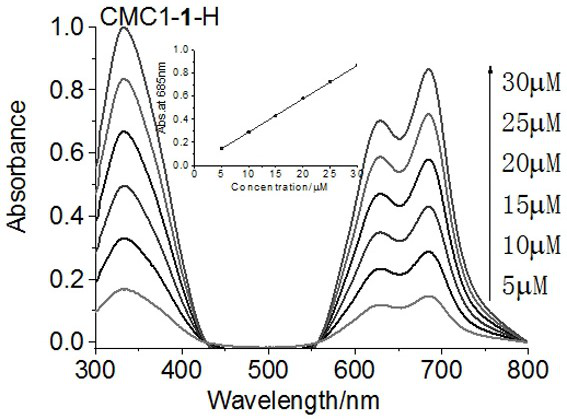
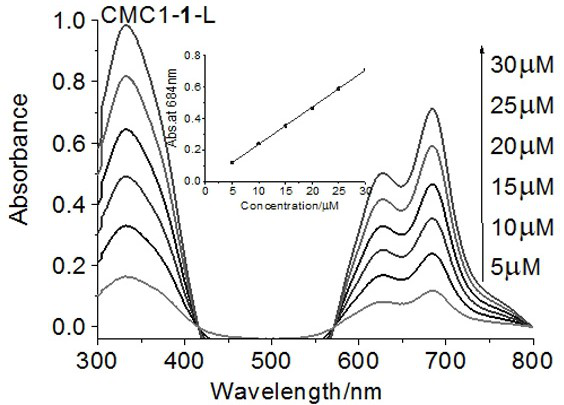
[0035] Dissolve 13.5 mg of 1-hydroxybenzotriazole (HOBt, 0.1 mmol) in 0.5 mL of dimethylsulfoxide to obtain HOBt solution, 19.2 mg of 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide hydrochloride (EDCI, 0.1 mmol) was dissolved in 0.5 mL of water to obtain EDCI solution, 7.09 mg of zinc aminophthalocyanine (0.01 mmol) was dissolved in 0.5 mL of dimethyl sulfoxide to obtain phthalocyanine compound solution, spare. Then 175.2 mg carboxymethyl chitosan (MW=50 kDa, 0.7 mmol repeating unit number) was dissolved in 10 mL of 1% hydrochloric acid solution, HOBt solution and EDCI solution were added successively, and stirred at room temperature for 1 h to activate the carboxyl group . The phthalocyanine compound solution was slowly added dropwise to the mixture, and the reaction was stirred overnight at room temperature under the protection of nitrogen. Concentrate to dryness after the reaction, add 150 mL of water to dissolve, use sand core funnel to filter repeatedly 3 times, concent...
Embodiment 2
[0038] The consumption of carboxymethyl chitosan in embodiment 1 is adjusted 250.3mg, makes the mol ratio of its repeating unit number and phthalocyanine be 100:1, all the other steps are with embodiment 1, make phthalocyanine and average molecular weight is 50 kDa The conjugate of carboxymethyl chitosan, the yield is 18.4%, the phthalocyanine content is 0.31%.
[0039] Change the product from -COONa to -COOH, and the corresponding infrared spectrum characteristic peak is: FT-IR (cm -1 ): 3373 (-OH, -NH, -NH 2 ), 2888 (-CH, -CH 2 ), 1727 (-COOH, C=O), 1618 (amide I, C=O), 1320 (C-N, -CH 3 ), 1061 (C-O-C).
Embodiment 3
[0041] The molecular weight of carboxymethyl chitosan in embodiment 1 is adjusted to 170 kDa, and consumption is adjusted to 150.2mg, makes the mol ratio of its repeating unit number and phthalocyanine be 60:1, all the other steps are the same as embodiment 1, make phthalocyanine The yield of the conjugate of cyanine and carboxymethyl chitosan with an average molecular weight of 170kDa is 27.1%, and the content of phthalocyanine is 1.50%.
[0042] Change the product from -COONa to -COOH, and the corresponding infrared spectrum characteristic peak is: FT-IR (cm -1 ): 3362 (-OH, -NH, -NH 2 ), 2890 (-CH, -CH 2 ), 1728 (-COOH, C=O), 1625 (amide I, C=O), 1374 (C-N, -CH 3 ), 1058 (C-O-C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com