Sh2b adapter protein 3 for the prediction of bone marrow response and immune response
A technology of SH2B3, immune response, applied in the determination/inspection of microorganisms, biochemical equipment and methods, complex mathematical operations, etc., can solve problems such as unsolved problems, and achieve the effect of reducing costs and adverse drug reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0115] CD133 in myocardium + Purified autologous bone marrow stem cell (BMSC) transplantation has been investigated as an adjunct strategy to coronary artery bypass grafting (CABG) revascularization in acute myocardial ST-segment elevation infarction (STEMI) and sequentially in coronary 3-vessel disease Restoration of left ventricular cardiac function after deterioration of left ventricular ejection fraction (LVEF) following acute PCI and secondary CABG revascularization. Previous safety and efficacy (Phase I, IIa, IIb) trials have demonstrated enhancement of left ventricular ejection fraction (LVEF) and adjuvant CD133 + Clinical safety of BMSC therapy on coronary revascularization. Design a randomized double-blind placebo-controlled clinical trial to evaluate clinical safety, efficacy and biomarkers for interventional CD133 + Identification of CD133 in BMSC transplantation + Cardiac repair mechanisms associated with bone marrow stem cells.
[0116] Conduct a randomized do...
example 2
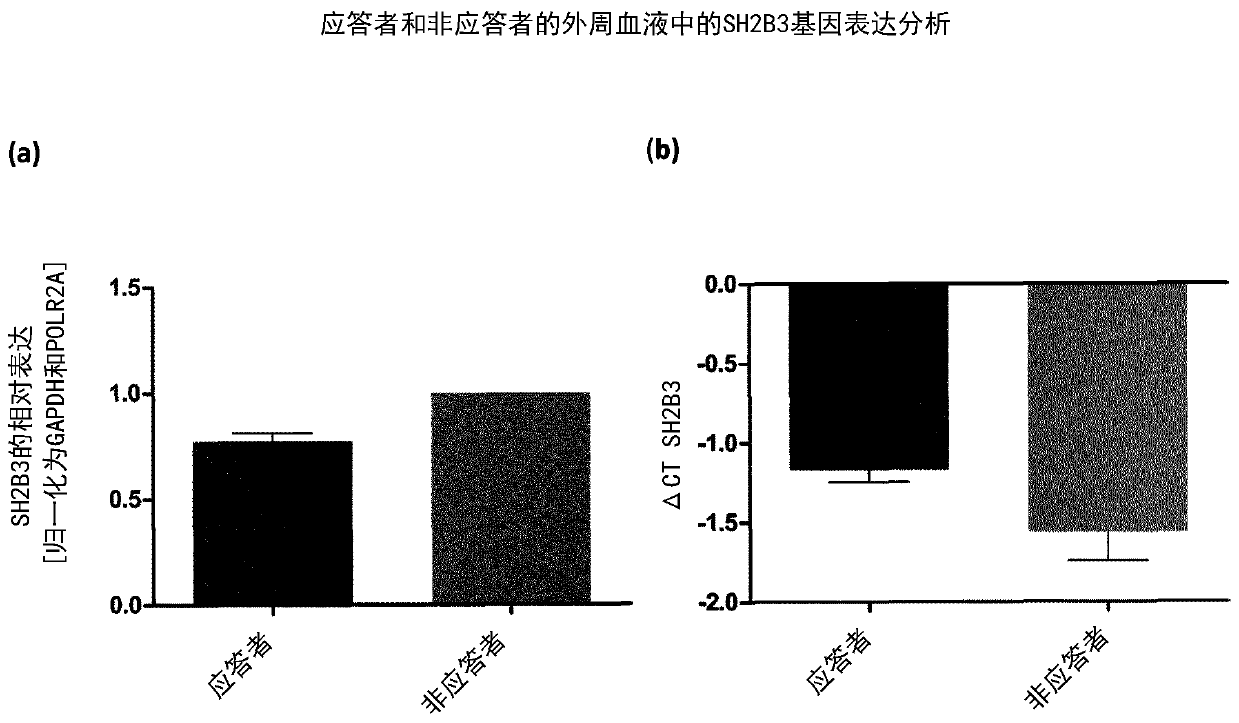
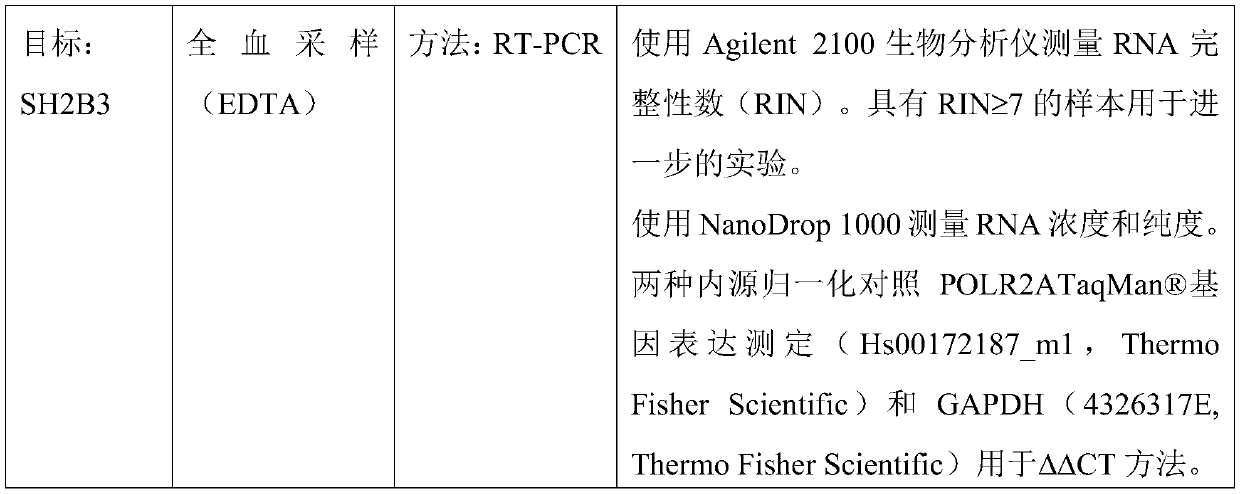
[0126] Measurement of SH2B3 gene expression was performed by whole blood sampling followed by RT-PCR.
[0127] Table 1: Measurement of SH2B3 gene expression by RT-PCR
[0128]
example 3
[0130]Native samples of peripheral blood (EDTA blood) were used for quantitative real-time-PCR using LightCycler 480II (Roche Deutschland Holding GmbH).
[0131] RNA was isolated from 1 ml whole blood aliquots (stored at -80°C) using the GeneJET Stabilized and Fresh Whole Blood RNA Kit (Thermo Fisher Scientific). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Using the StepOnePlusRT PCR system (Applied Biosystems TM ) for RT-PCR. Using cDNA (30ng per reaction), Universal PCR Master Mix (Thermo Fisher Scientific) and SH2B3 GeneExpression Assay (Hs01081959_g1, Thermo Fisher Scientific). Three replicates of the process were performed. To calculate the relative expression ratio of SH2B3, the ΔΔCT method was employed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com