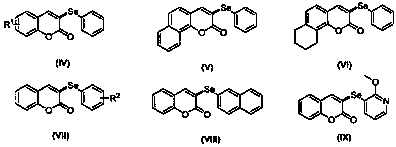
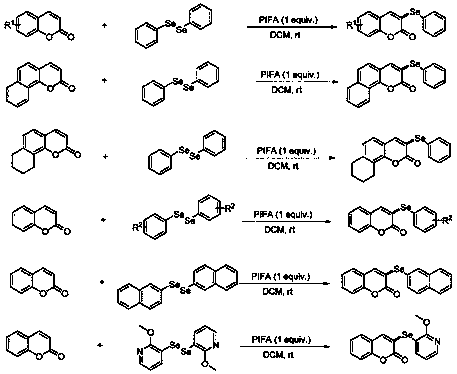
Preparation method of 3-selenocoumarin compound
A technology for substituting coumarins and coumarins, which is applied in the field of organic synthesis to achieve the effects of high yield, simple operation and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add coumarin (0.2mmol), 1,2-diphenyl diselenide (0.4 mmol), PIFA (0.4mmol), TMSN 3 (Azidotrimethylsilane) (0.4 mmol) and toluene (toluene) (2.0 mL), stirred at room temperature. TLC tracking detection reaction. After 12 hours, the starting material had been partially converted. Water and dichloromethane were added to the reaction system, and the organic layer was separated. The aqueous layer was washed twice with dichloromethane, all organic layers were combined and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (10% ethyl acetate petroleum ether solution) to obtain 45.1 mg of product with a yield of 75%. The reaction process is shown in the following formula:
[0039]
[0040] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0041] 1 H NMR (500MHz, DMSO-d 6 )δ7.70–7.68(m,2H),7.56–7.47(m,5H),7.42(s,1H),7.40(d,J=8.3Hz,1H),7.29...
Embodiment 2
[0043] Add coumarin (0.2mmol), 1,2-diphenyl diselenide (0.4 mmol), PIFA (0.4mmol), TMSN 3(0.4 mmol) and MeCN (acetonitrile) (2.0 mL), stirred at room temperature. TLC tracking detection reaction. After 0.5 hour, the starting material was completely converted. Water and dichloromethane were added to the reaction system, and the organic layer was separated. The aqueous layer was washed twice with dichloromethane, all organic layers were combined and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (10% ethyl acetate petroleum ether solution) to obtain 53.6 mg of product with a yield of 89%. The reaction process is shown in the following formula:
[0044]
[0045] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0046] 1 H NMR (500MHz, DMSO-d 6 )δ7.70–7.68(m,2H),7.56–7.47(m,5H),7.42(s,1H),7.40(d,J=8.3Hz,1H),7.29–7.25(m,1H)ppm; 13 C NMR ...
Embodiment 3
[0048] Add coumarin (0.2mmol), 1,2-diphenyl diselenide (0.4 mmol), PIFA (0.4mmol), TMSN 3 (0.4mmol) and DCM (2.0mL), stirred at room temperature. TLC tracking detection reaction. After 0.5 hour, the starting material was completely converted. Water and dichloromethane were added to the reaction system, and the organic layer was separated. The aqueous layer was washed twice with dichloromethane, all organic layers were combined and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (10% ethyl acetate petroleum ether solution) to obtain 59.0 mg of product with a yield of 98%. The reaction process is shown in the following formula:
[0049]
[0050] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0051] 1 H NMR (500MHz, DMSO-d 6 )δ7.70–7.68(m,2H),7.56–7.47(m,5H),7.42(s,1H),7.40(d,J=8.3Hz,1H),7.29–7.25(m,1H)ppm; 13 C NMR (126MHz, DMSO-d ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com