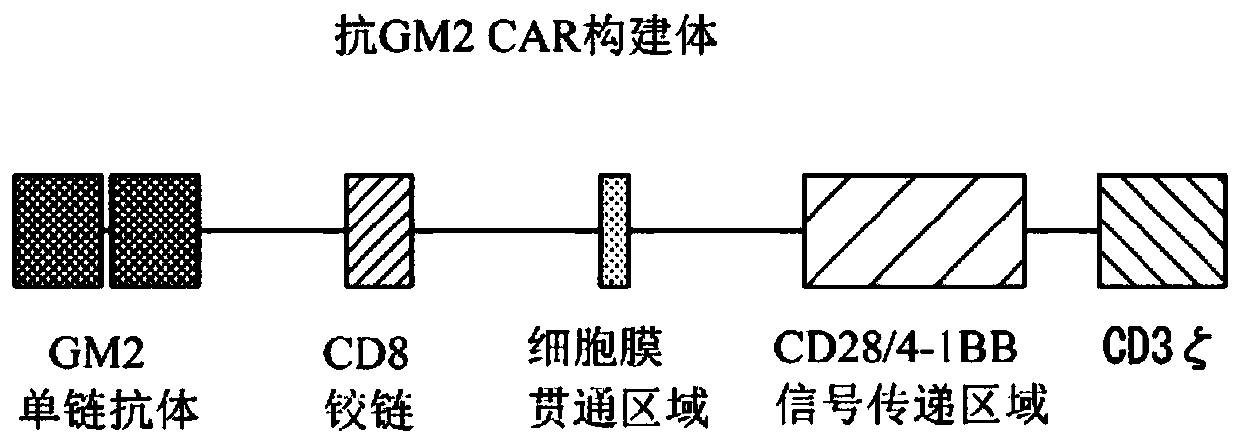
Chimeric antigen receptor
A chimeric antigen receptor, antigen technology, applied in the direction of antibodies, antibody medical components, antibody mimetics/scaffolds, etc., can solve the problems of solid tumors that have not been shown to be effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
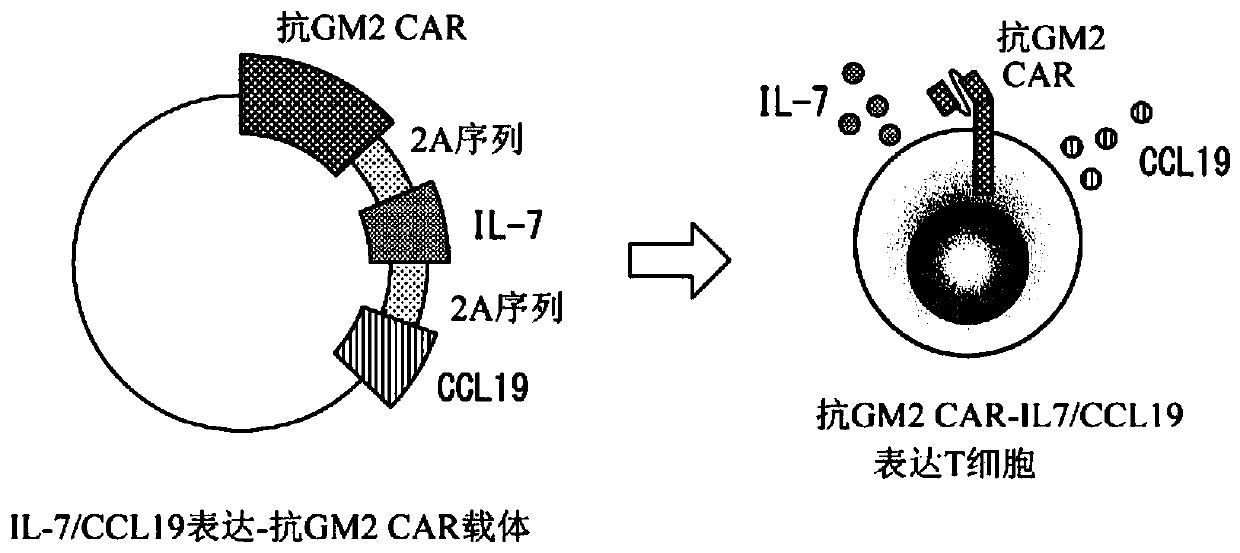
[0251] [Example 1] Production of anti-GM2 CAR expressing T cells expressing IL-7 and CCL19 (selection of immune function promoting factors of T cells)
[0252] There are at least several hundred kinds of molecules capable of controlling the functions of T cells in the living body. The present inventors selected IL-7 and CCL19 as immune function promoting factors for improving the anti-tumor effect in CAR-T cells from a huge combination.
[0253] It should be noted that the aforementioned IL-7 is a cytokine necessary for the survival of T cells, and is produced by non-hematopoietic cells such as stromal cells present in bone marrow, thymus, and lymphoid organ tissues. On the other hand, almost no IL-7 producing ability was found in T cells.
[0254] In addition, the above-mentioned CCL19 is mainly produced by dendritic cells or macrophages in lymph nodes, and has a function of inducing the migration of T cells, B cells, and mature dendritic cells via CCR7 as its receptor.
[...
Embodiment 2
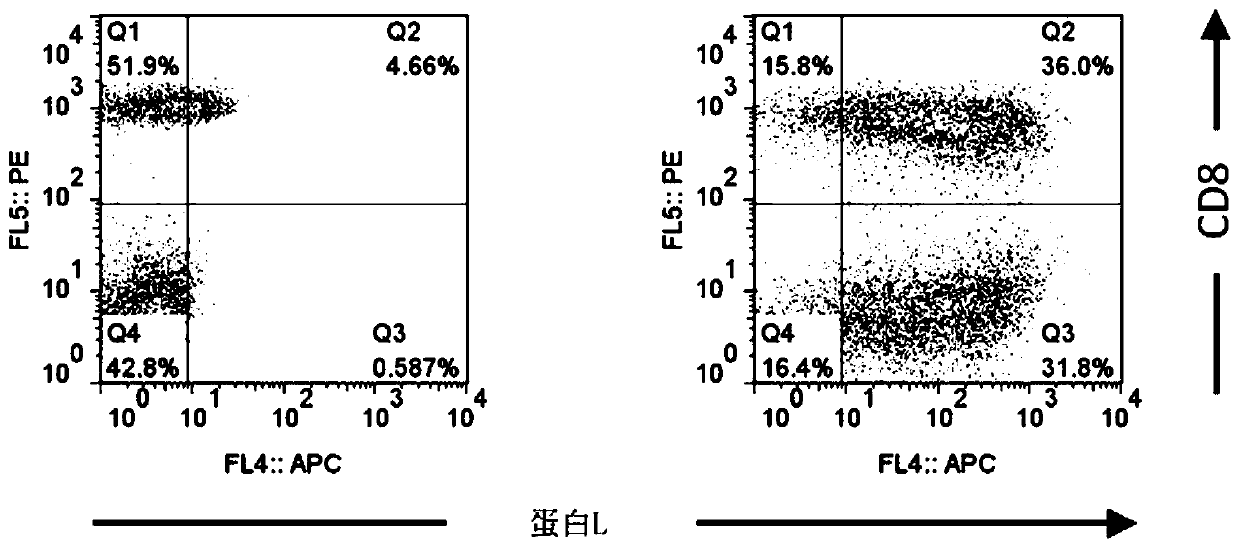
[0267] [Example 2] CAR expression measurement by flow cytometry (flow cytometry analysis)
[0268] Analysis of the expression level of CAR that recognizes GM2 as an antigen was performed by two-color flow cytometry analysis. The prepared anti-GM2 CAR-IL-7 / CCL19-expressing T cells were mixed with biotinylated protein L (manufactured by GenScript), allophycocyanin (APC)-labeled streptavidin (manufactured by Affymetrix), and APC-labeled Anti-CD8 monoclonal antibody (manufactured by Affymetrix) was reacted for staining. EC800 (manufactured by Sony Corporation) was used for the flow cytometer, and FlowJo software (manufactured by Tree Star Corporation) was used for data analysis.
[0269] show the result in figure 2 . The left picture shows cells without CAR gene introduction, and the right picture shows the results of anti-GM2 CAR-IL-7 / CCL19 expressing T cells. In addition, the numerical value in a figure shows the percentage of each population. Such as figure 2 As shown, ...
Embodiment 3
[0270] [Example 3] Production of IL-7 and CCL19
[0271] (Determination of IL-7 and CCL19 concentrations in the culture supernatant of anti-GM2 CAR-IL-7 / CCL19 expressing T cells)
[0272] The culture supernatant of anti-GM2 CAR-IL-7 / CCL19 expressing T cells or non-gene-introduced cells on the 7th day of the above-mentioned culture was recovered, and commercially available ELISA kits (manufactured by Peprotech and R&D systems, respectively) were used to detect the The production of IL-7 and CCL19 by anti-GM2 CAR-IL-7 / CCL19 expressing T cells was investigated.
[0273] [result]
[0274] The results are shown in image 3 . Such as image 3 As shown, in the culture supernatant (GM2CAR) of anti-GM2 CAR-IL-7 / CCL19 expressing T cells, IL-7 was detected to be more than 300 pg / ml, and CCL19 was detected to be more than 2000 pg / ml. From these results, it was confirmed that the anti-GM2CAR-IL-7 / CCL19-expressing T cells expressed IL-7 and CCL19, and the expressed IL-7 and CCL19 were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com