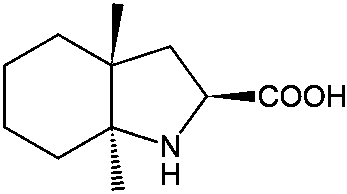
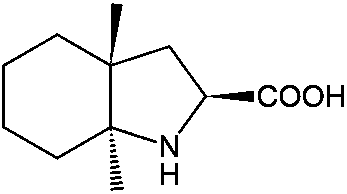
Resolution method for racemate of trandolapril intermediate
A technology of trandolapril and intermediates, applied in the field of biocatalysis, can solve complex problems such as racemization, and achieve the effect of short reaction time, mild reaction conditions, and high-efficiency resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Utilize the ammonium acetate buffer solution in the beaker to configure the reaction solution with a pH of 8.0, weigh it according to the mass ratio of Trumplidol intermediate and Pichia pastoris lipase as 2:1, add it to the beaker, and then place the beaker In a constant temperature water bath at 25°C, react for 6 hours, add a certain amount of hydrochloric acid to terminate the reaction, transfer all the reaction solution to a volumetric flask, use mobile phase to constant volume, dilute a certain number of times, and then filter through a microporous organic filter membrane , and finally use a high-performance liquid chromatograph to analyze and detect that the conversion rate is 99.8%.
[0023] Example 1
[0024] Utilize the ammonium acetate buffer solution in the beaker to configure the reaction solution with a pH of 8.0, weigh it according to the mass ratio of Trumplidol intermediate and Pichia pastoris lipase as 2:1, add it to the beaker, and then place the beake...
Embodiment 2
[0026] Utilize the ammonium acetate buffer solution in the beaker to configure the reaction solution with a pH of 8.5, weigh it according to the mass ratio of Trumplidol intermediate and Pichia pastoris lipase as 4:1, add it to the beaker, and then place the beaker React for 4 hours in a constant temperature water bath at 30°C, add a certain amount of hydrochloric acid to terminate the reaction, transfer the entire reaction solution to a volumetric flask, dilute to a certain volume with mobile phase, and then filter through a microporous organic filter membrane , and finally use a high-performance liquid chromatograph to analyze and detect and measure the conversion rate to be 97.6%.
Embodiment 3
[0028] Utilize the ammonium acetate buffer solution in the beaker to configure the reaction solution with a pH of 7.7, weigh it according to the mass ratio of the intermediate of Trumplidol and Pichia pastoris lipase as 3:1, add it to the beaker, and then place the beaker React for 3 hours in a constant temperature water bath at 27°C, add a certain amount of hydrochloric acid to terminate the reaction, transfer the entire reaction solution to a volumetric flask, dilute to a certain volume with mobile phase, and then filter through a microporous organic filter membrane , and finally use a high-performance liquid chromatograph to analyze and detect that the conversion rate is 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com