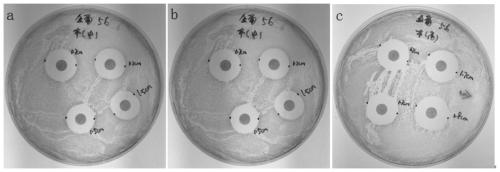
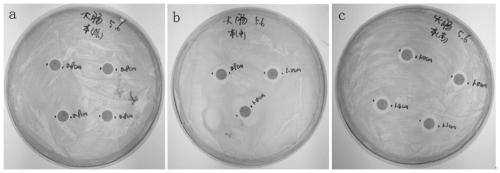
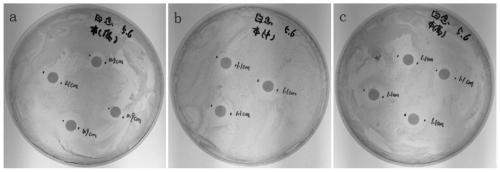
Virus delivery culture medium, and preparation method and application thereof
A culture medium and virus technology, applied in the field of culture liquid, can solve the problems of high price, uneven performance, etc., and achieve the effects of low production cost, maintaining integrity, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A virus transport culture medium is prepared by the following method:
[0036] 1) Prepare 4× Hank’s balanced salt solution according to the following formula:
[0037]
[0038] 2) Prepare the virus delivery medium according to the table below:
[0039] Table 1. The virus delivery medium preparation table of embodiment 1
[0040] component name stock solution concentration Dosage Final concentration Hank's Balanced Salt Solution 4× 125mL 0.5× gelatin 5% 20mL 1g / L BSA 10% 25mL 2.5g / L sucrose 5% 20mL 1g / L L-cysteine 0.015mmol / mL 6.6mL 0.1mM / L L-glutamic acid 0.015mmol / mL 6.6mL 0.1mM / L Hepes 1M / L 5mL 5mM / L Amphotericin B 2.5mg / mL 4mL 10mg / L Polymyxin B 2mg / mL 5mL 10mg / L Gentamicin 10mg / mL 1mL 10mg / L water / Set volume to 1L /
[0041] Among them, 4 × Hank's balanced salt solution, sucrose, BSA solution, L-cysteine, L-glutamic acid, Hepes,...
Embodiment 2
[0043] A virus delivery medium, the difference from Example 1 is that the components and proportioning ratio are shown in the following table:
[0044] Table 2. The virus delivery medium preparation table of embodiment 2
[0045] component name stock solution concentration Dosage (Preparation 1L) Final concentration Hank's Balanced Salt Solution 4× 250mL 1× gelatin 5% 50mL 2.5g / L BSA 10% 50mL 5g / L sucrose 5% 50mL 2.5g / L L-cysteine 0.015mmol / mL 10mL 0.150mM / L L-glutamic acid 0.015mmol / mL 10mL 0.150mM / L Hepes 1M 10mL 10mM / L Amphotericin B 2.5mg / mL 8mL 20mg / L Polymyxin B 2mg / mL 10mL 20mg / L Gentamicin 10mg / mL 2mL 20mg / L water / Set volume to 1L /
Embodiment 3
[0047] A virus delivery medium, the difference from Example 1 is that the components and proportioning ratio are shown in the following table:
[0048] Table 3. Example 3 virus delivery medium preparation table
[0049] component name stock solution concentration Dosage (Preparation 1L) Final concentration Hank's Balanced Salt Solution 4× 500mL 2× gelatin 5% 100mL 5g / L BSA 10% 100mL 10g / L sucrose 5% 100mL 5g / L L-cysteine 0.015mmol / mL 20mL 0.3mM / L L-glutamic acid 0.015mmol / mL 20mL 0.3mM / L Hepes 1M 20mL 20mM / L Amphotericin B 2.5mg / mL 16mL 40mg / L Polymyxin B 2mg / mL 20mL 40mg / L Gentamicin 10mg / mL 4mL 40mg / L water / Set volume to 1L /
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com