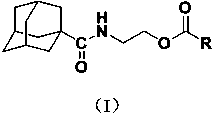
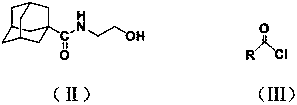
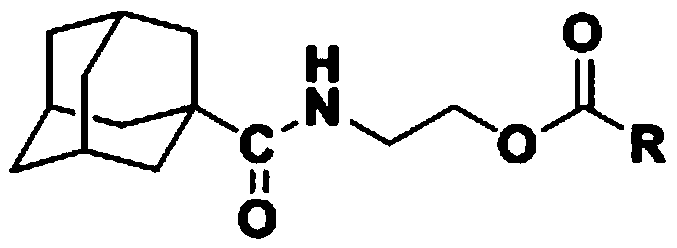
2-(1-adamantanecarboxamido) ethyl formate compound and preparation method and application thereof
A technology for adamantanecarboxamide and ester compounds, which is applied in the field of 2-(1-adamantanecarboxamido)ethyl formate and its preparation and application, and can solve the problems that have not been found in the research of structure and biological activity. Literature reports and other issues, to achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of compound Ia (R = phenyl):
[0028] Dissolve 1.5 mmol of benzoyl chloride in 5 mL of tetrahydrofuran to prepare a tetrahydrofuran solution (5 mL) of benzoyl chloride (1.5 mmol).
[0029] The intermediate N-2-hydroxyethyl-1-adamantanecarboxamide (0.335g, 1.5mmol) was mixed with tetrahydrofuran (10mL) and the acid-binding agent triethylamine (1.8mmol), stirred and dissolved, and placed in an ice bath A solution of benzoyl chloride (1.5 mmol) in tetrahydrofuran (5 mL) was slowly added dropwise. After the dropwise addition was completed, react at room temperature for 3 h, then filter to remove the hydrochloride of triethylamine generated by the reaction, remove the solvent tetrahydrofuran by rotary evaporation of the filtrate, and obtain a white solid through column chromatography separation of the obtained rotary evaporation residue (eluent is volume A mixture of ethyl acetate and petroleum ether at a ratio of 1:5) is benzoic acid-2-(1-adamantanecarb...
Embodiment 2
[0032] Example 2 Synthesis of compound Ib (R=2-chlorophenyl):
[0033] The intermediate N-2-hydroxyethyl-1-adamantanecarboxamide (0.335g, 1.5mmol) was mixed with tetrahydrofuran (12mL) and the acid-binding agent triethylamine (2.7mmol), stirred and dissolved, and placed in an ice bath A solution of 2-chlorobenzoyl chloride (2.0 mmol) in tetrahydrofuran (6 mL) was slowly added dropwise. After the dropwise addition was completed, react at room temperature for 4 h, then filter to remove the hydrochloride of triethylamine generated by the reaction, remove the solvent tetrahydrofuran by rotary evaporation of the filtrate, and obtain a white solid through column chromatography separation of the obtained rotary evaporation residue (eluent is volume A mixture of ethyl acetate and petroleum ether at a ratio of 1:4) is 2-(1-adamantanecarboxamido)ethyl 2-chlorobenzoate, and the calculated yield is 65.3%. m.p.:120~123℃;
[0034] 1 H NMR (500MHz, CDCl 3 )δ7.83(dd,J=7.5,1.0Hz,1H),7.49–7...
Embodiment 3
[0036] Example 3 Synthesis of compound Ic (R=3-chlorophenyl):
[0037] The intermediate N-2-hydroxyethyl-1-adamantanecarboxamide (0.335g, 1.5mmol) was mixed with tetrahydrofuran (12mL) and the acid-binding agent triethylamine (2.7mmol), stirred and dissolved, and placed in an ice bath A solution of 3-chlorobenzoyl chloride (2.0 mmol) in tetrahydrofuran (6 mL) was slowly added dropwise. After the dropwise addition was completed, react at room temperature for 3 h, then filter to remove the hydrochloride of triethylamine generated by the reaction, remove the solvent tetrahydrofuran by rotary evaporation of the filtrate, and obtain a white solid through column chromatography separation of the obtained rotary evaporation residue (eluent is volume A mixture of ethyl acetate and petroleum ether at a ratio of 1:3) is 2-(1-adamantanecarboxamido)ethyl 3-chlorobenzoate, and the calculated yield is 52.5%. m.p.:127~130℃;
[0038] 1 H NMR (500MHz, CDCl 3 )δ7.94(s,1H),7.86(d,J=7.5Hz,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com