Chiral Tetrastyryltetramines for Enantiomeric Identification and Purity Analysis of Chiral Carboxylic Acids
A technology of chiral carboxylic acid and chiral amine, which is applied in the field of chemical analysis, can solve the problem of low accuracy of enantiomer purity analysis of chiral carboxylic acid, achieve high sensitivity, high accuracy, and improve the effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Chiral fluorescence analysis reagent I (in general formula I, R 1 = cyclohexyl; R 2 = methyl) synthesis:
[0041]
[0042]
[0043]Add the compound of formula II (0.05g-2g), anhydrous potassium carbonate (0.01g-5.0g), S-1-cyclohexylethylamine (0.06-6.0g) and tetrahydrofuran (1mL-100mL) into a round bottom flask , heated and stirred at 70-100°C for 4h-20h, evaporated to remove the solvent, and the remaining solid was purified by column chromatography (aluminum oxide, eluent: methanol / dichloromethane) to obtain the compound described in formula I , the yield is greater than 60%.
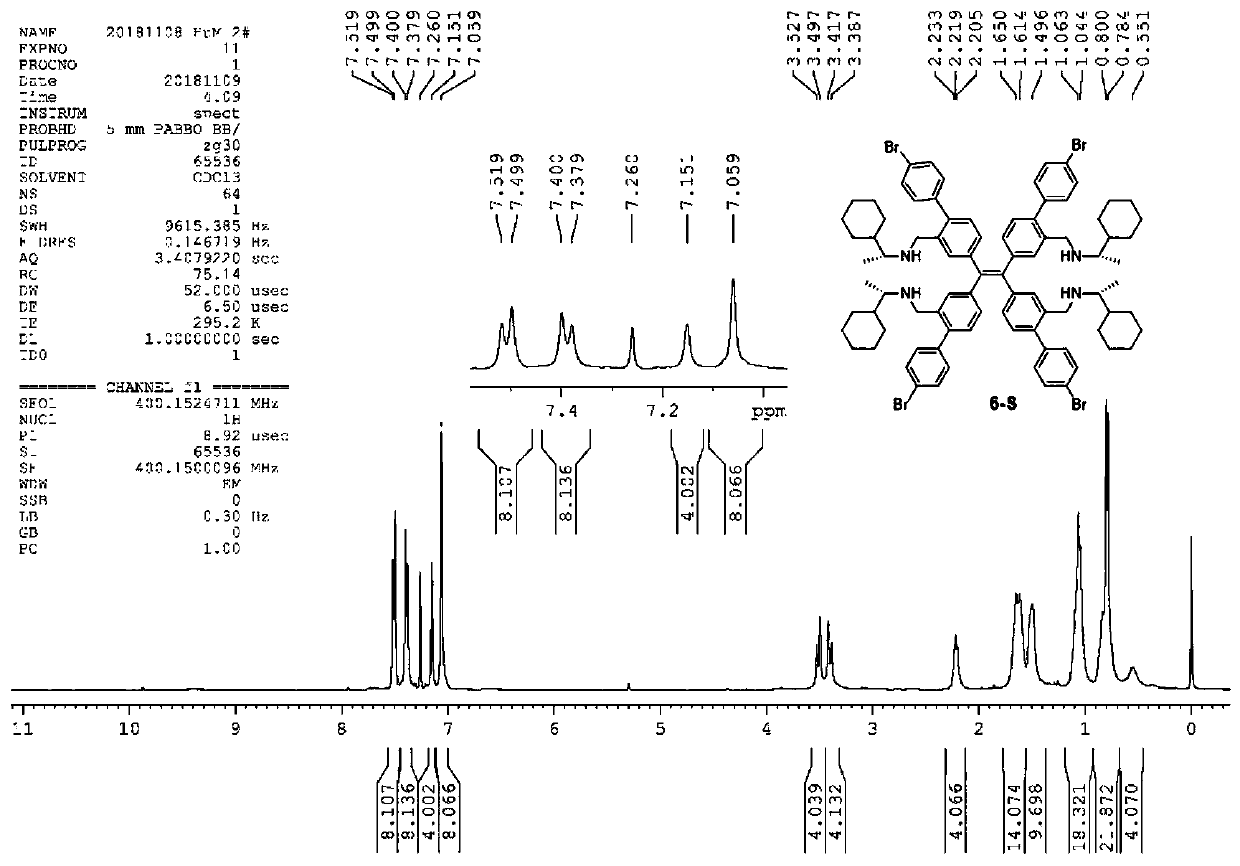
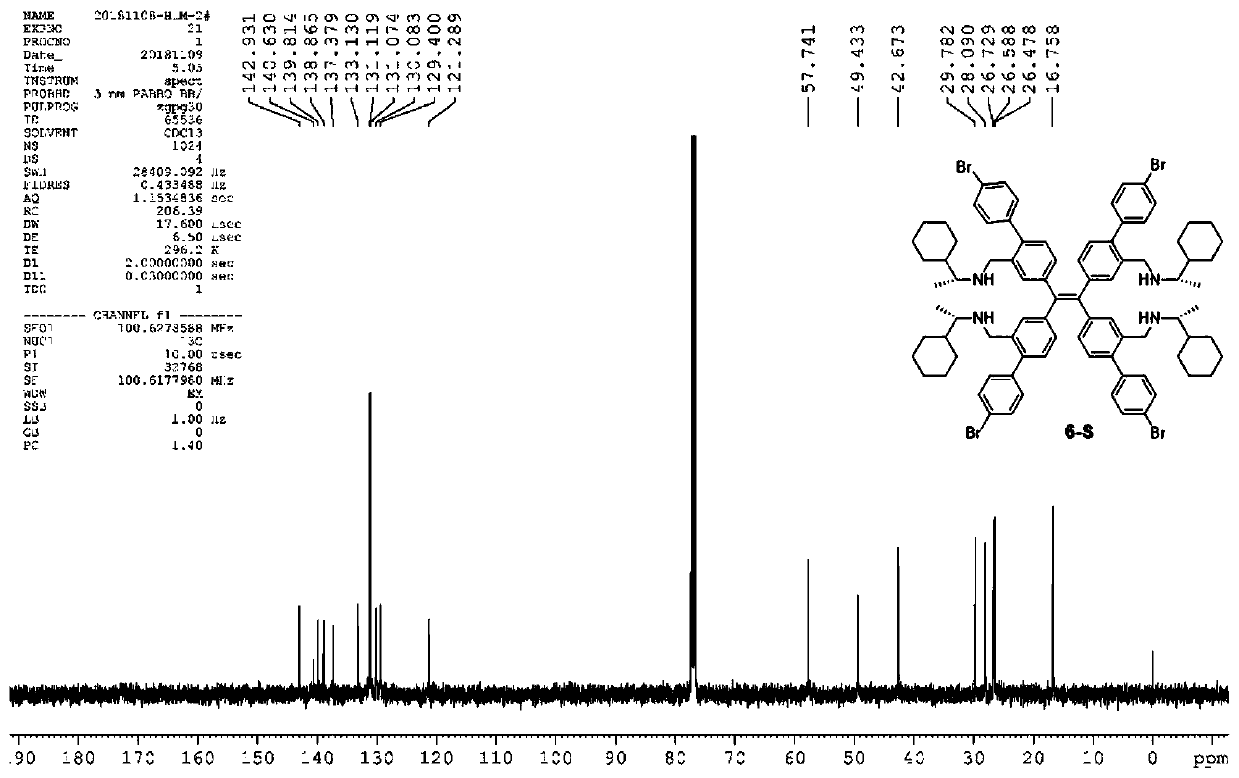
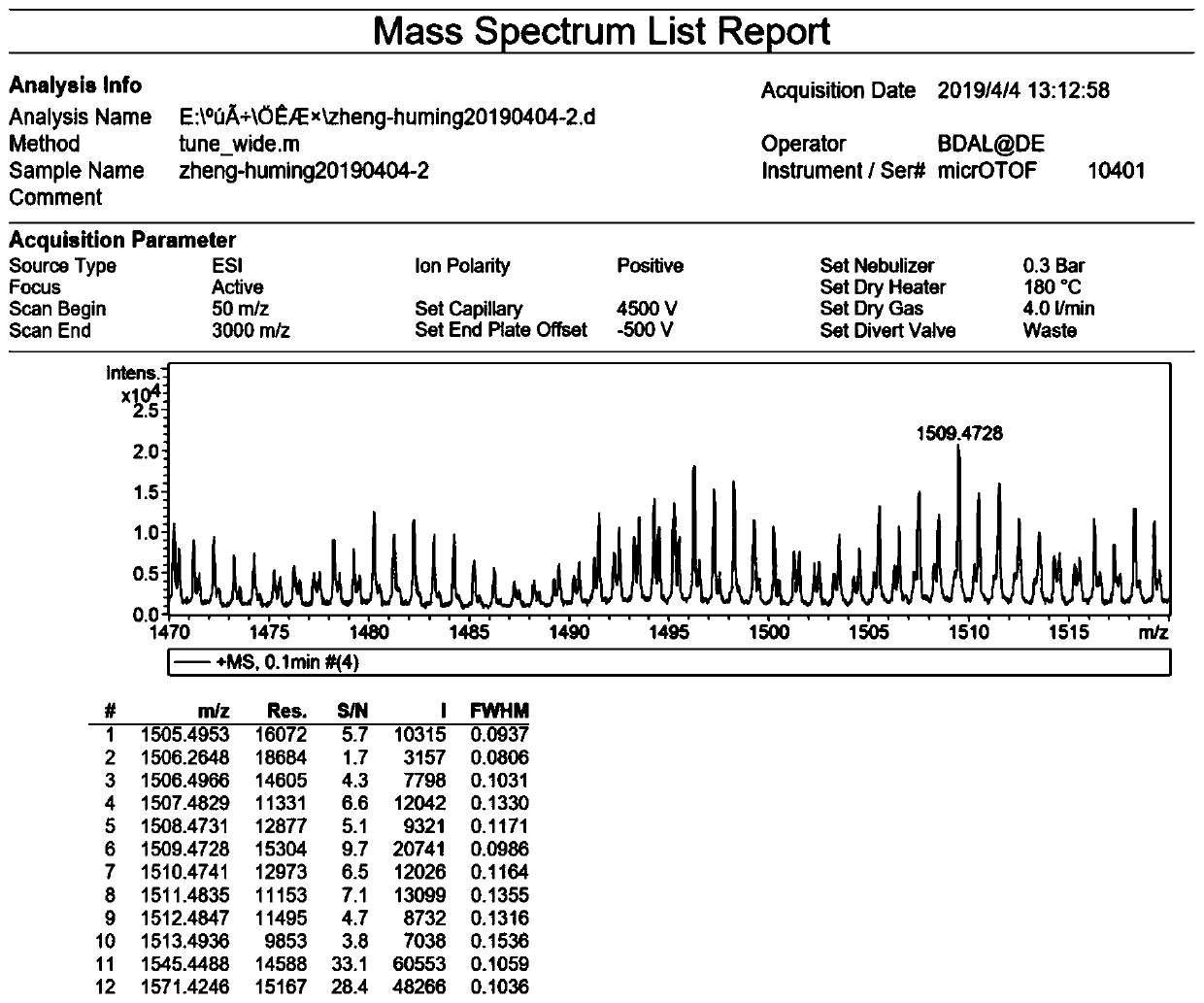
[0044] Formula I compound in CDCl 3 middle 1 H-NMR spectrum see figure 1 , 13 C-NMR spectrum see figure 2 , HRMS spectrum see image 3 .
Embodiment 2
[0046] The compound prepared in Example 1 (in general formula I, R 1 = cyclohexyl; R 2 =methyl; S-configuration) was dissolved in acetone to make 1.0×10 -3 M's solution. In three 5mL glass bottles, add 0.04mL of the prepared solution above; then add 0.04mL of acetone, 0.04mL of 2.0×10 -3 M's D-p-toluoyl tartrate in acetone and 0.04mL 2.0×10 -3 M L-p-toluoyl tartrate acetone solution, after shaking well, add 3.92 ml of cyclohexane respectively. After mixing evenly, test the fluorescence spectrum of the solution in the three bottles and take a photo of the fluorescence under a 365nm portable ultraviolet lamp. It can be seen under ultraviolet light that the solution without p-toluoyl tartaric acid is yellow fluorescence, the solution with D-p-toluoyl tartaric acid is blue fluorescence, and the solution with L-p-toluoyl tartaric acid is green fluorescence. For spectrograms and fluorescence photos, see Figure 4 , Figure 4 Middle bold body 6 is the compound of formula I, an...
Embodiment 3
[0048] The compound prepared in Example 1 (in general formula I, R 1 = cyclohexyl; R 2 =methyl; S-configuration) was dissolved in acetone to make 1.0×10 -3 M's solution. In three 5mL glass bottles, add 0.04mL of the prepared solution above; then add 0.04mL of acetone, 0.04mL4.0×10 -3 M herbicide R-2,4-D acetone solution and 0.04mL 4.0×10 - 3 M's herbicide S-2,4-D acetone solution, shake well, add 3.92 ml of cyclohexane respectively. After mixing evenly, test the fluorescence spectrum of the solution in the three bottles and take a photo of the fluorescence under a 365nm portable ultraviolet lamp. It can be seen under ultraviolet light that the solution without 2,4-D is yellow fluorescence, the solution with R-2,4-D is green fluorescence, and the solution with S-2,4-D is very light yellow fluorescence. For spectrograms and fluorescence photos, see Figure 5 , Figure 5 The middle bold body 6 is the compound of formula I, and the bold body 18 is the herbicide 2,4-D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com