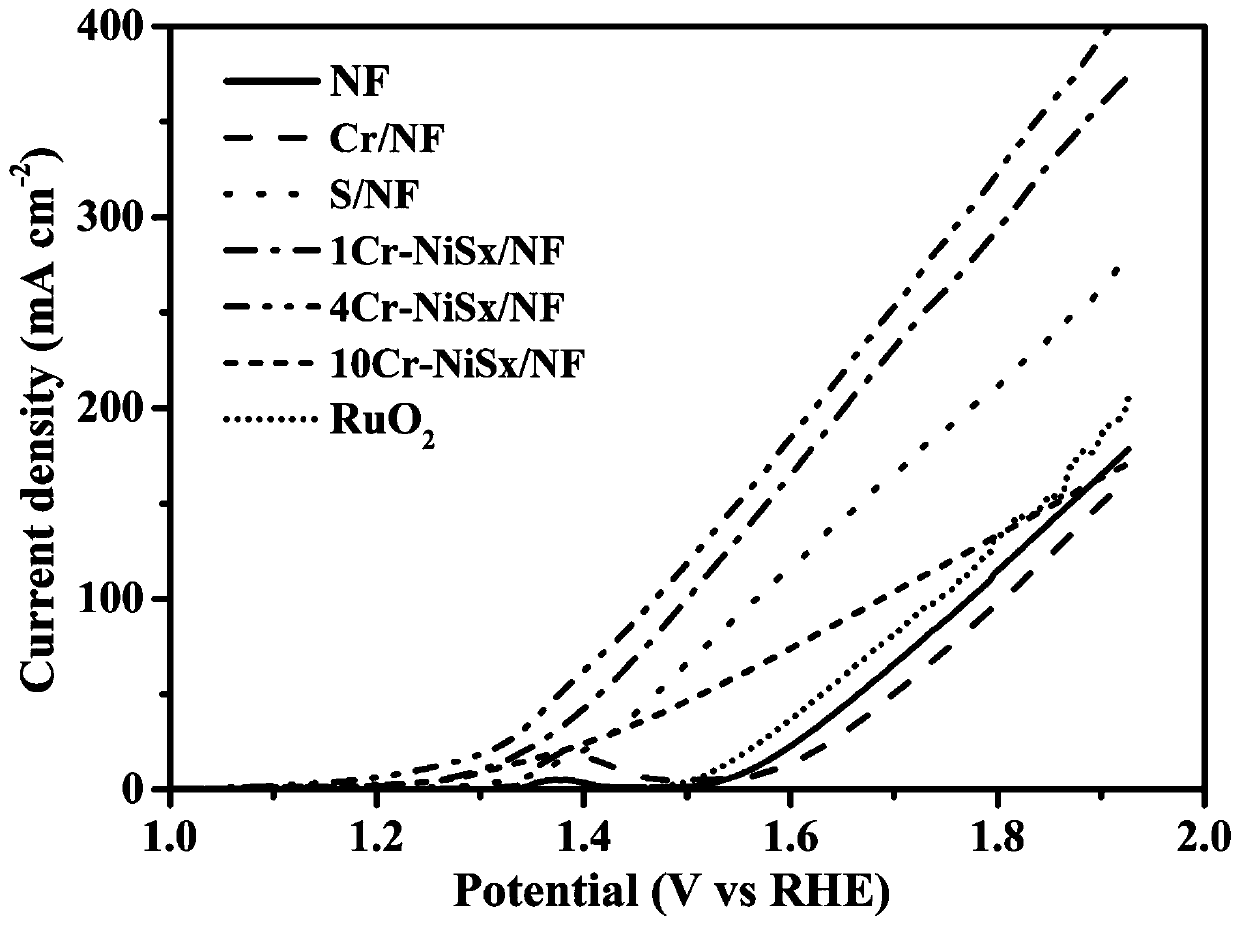
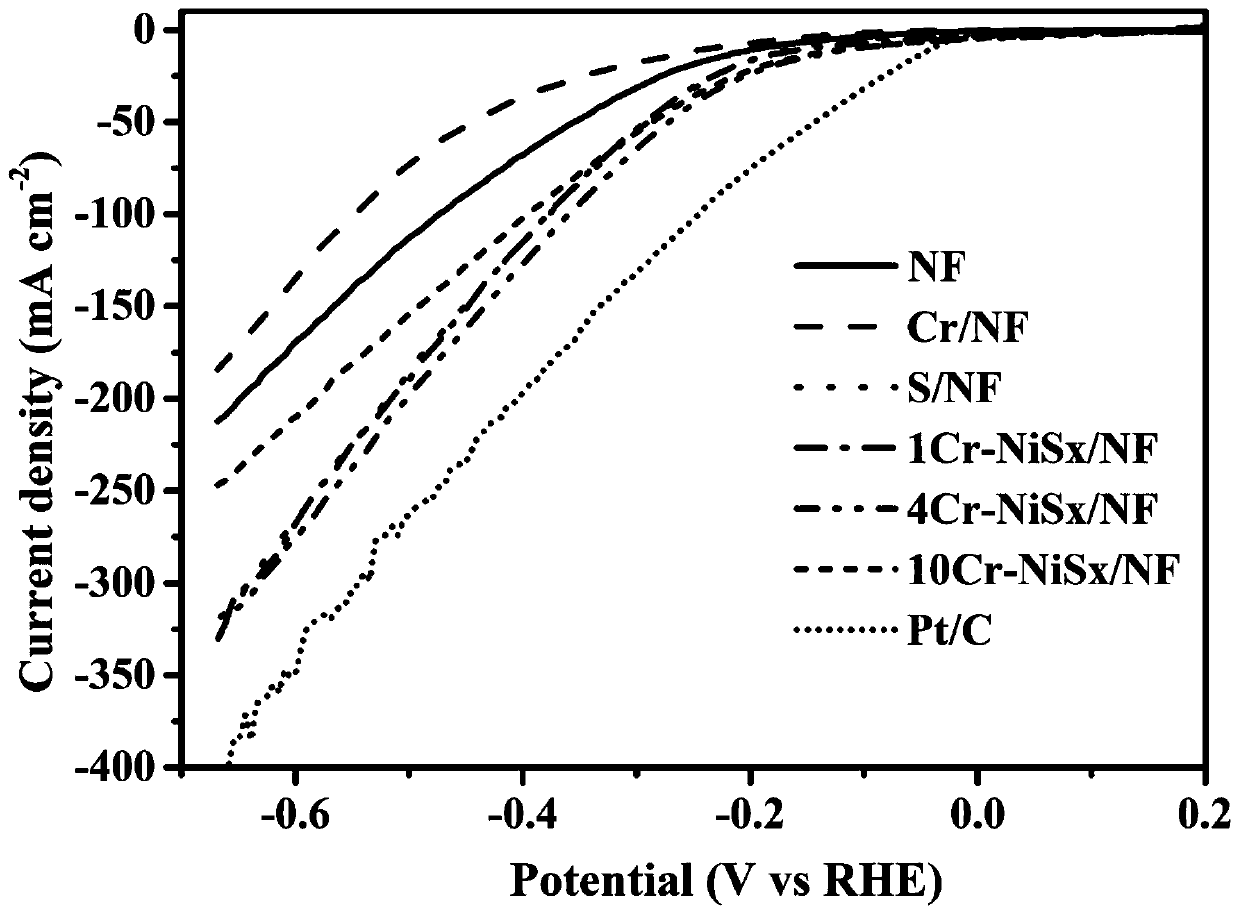
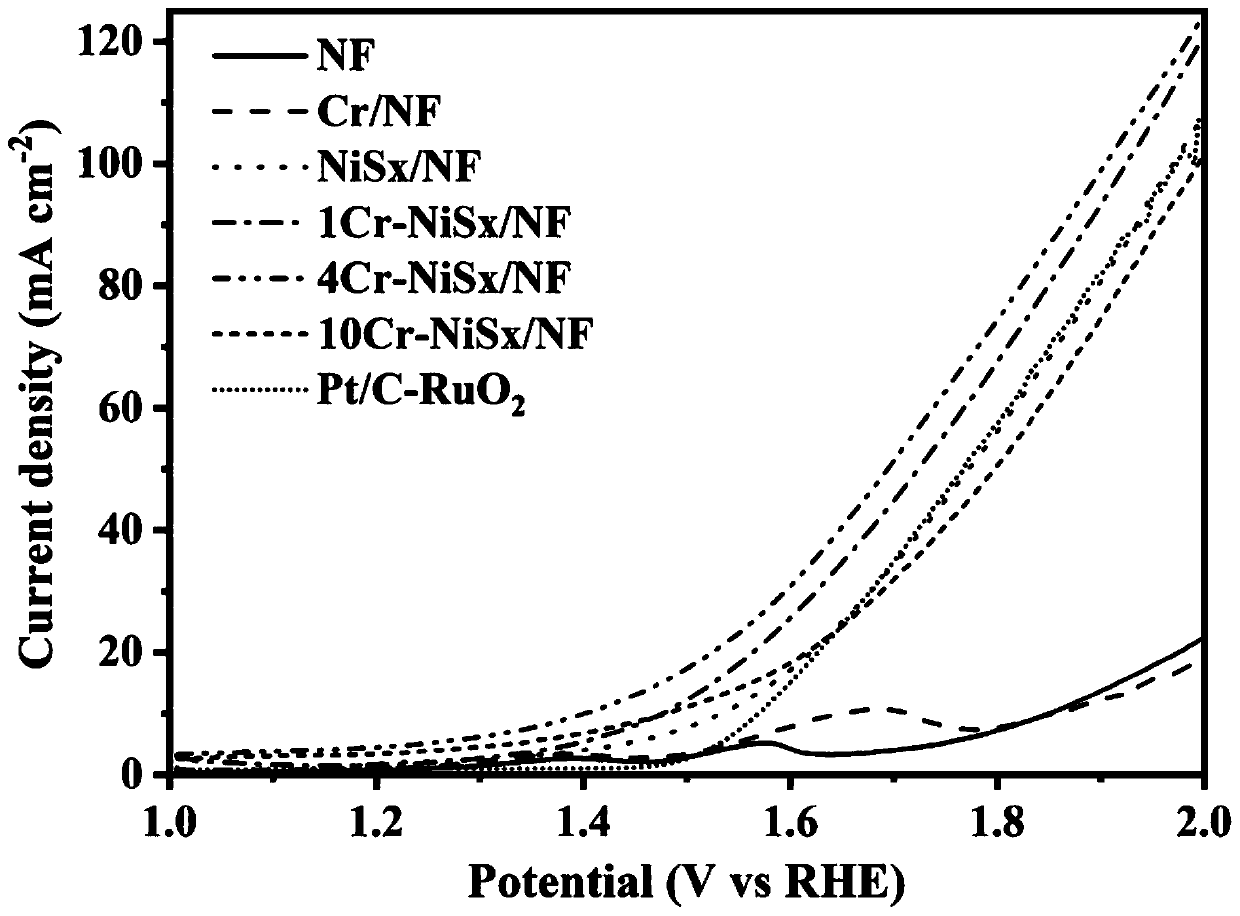
Dual-function fully-hydrolyzed hydroelectric catalyst and preparation method and application thereof
A catalyst, bifunctional technology, applied in the field of bifunctional electrocatalyst for total water splitting and its preparation, can solve problems such as instability of nickel-based catalyst, and achieve the effects of facilitating electron transfer, good chemical resistance, and speeding up resistance transmission.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A method for preparing a bifunctional full-scale water electrocatalyst, comprising:
[0023] S1. Uniformly mixing the sulfur-containing monomer with chromate and a solvent to prepare a mixed solution.
[0024] In the present invention, the sulfur-containing monomer includes at least one of thioacetamide and thiourea, and the chromate includes at least one of sodium chromate tetrahydrate and thioacetamide. In a preferred embodiment of the present invention, the sulfur-containing monomer is selected as thioacetamide, and the chromate is sodium chromate tetrahydrate. In a preferred embodiment of the present invention, in order to make the preparation process more environmentally friendly, the solvent is deionized water.
[0025] A sufficient amount of thioacetamide, sodium chromate tetrahydrate and deionized water were mixed and stirred for 1 hour to make them evenly mixed to obtain a mixed solution. Preferably, the mol ratio that participates in the thioacetamide of pre...
Embodiment 1
[0041] The preparation method of the bifunctional total water splitting electrocatalyst provided in this example is:
[0042] 1. Weigh thioacetamide (3g) and sodium chromate tetrahydrate (3.774g) and dissolve them in 35ml of deionized water, and stir for 1 hour to obtain a mixed solution with a concentration of 0.194g / ml.
[0043] 2. Soak nickel foam (NF; 40*20*2mm) in 3M dilute hydrochloric acid for ten minutes, then clean it with deionized water, then ultrasonicate it with acetone for half an hour, then ultrasonicate it with deionized water for half an hour, and finally use vacuum Dry in an oven at 60°C for later use.
[0044] 3. Put the mixed solution prepared in step 1 and the treated foamed nickel into the hydrothermal reaction kettle, tighten it, and put it in an oven at 180°C for 6 hours.
[0045] 4. After the reaction, 4Cr-NiSx / NF was obtained, which was washed several times with deionized water and ethanol.
[0046] 5. Dry 4Cr-NiSx / NF in a vacuum oven at 60° C. for ...
Embodiment 2
[0048] The preparation method of the bifunctional total water splitting electrocatalyst provided in this example is basically the same as that in Example 1, the only difference being:
[0049] Sodium chromate tetrahydrate consumption is 0.9435g. The concentration of the mixed solution was 0.113 g / ml. 1Cr-NiSx / NF was produced.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com