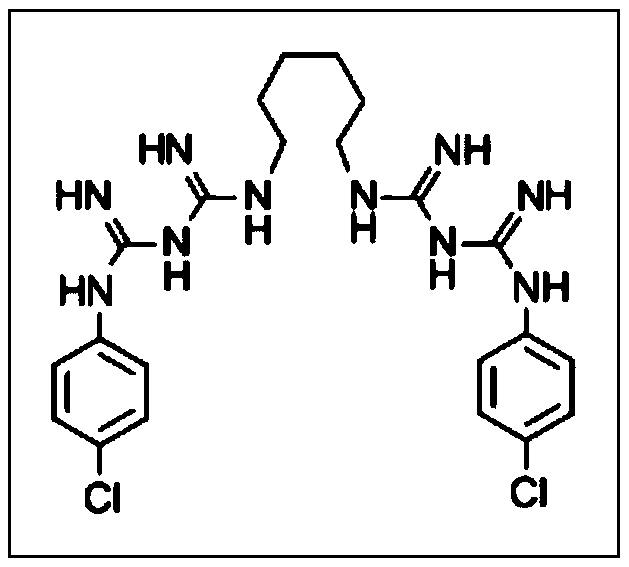
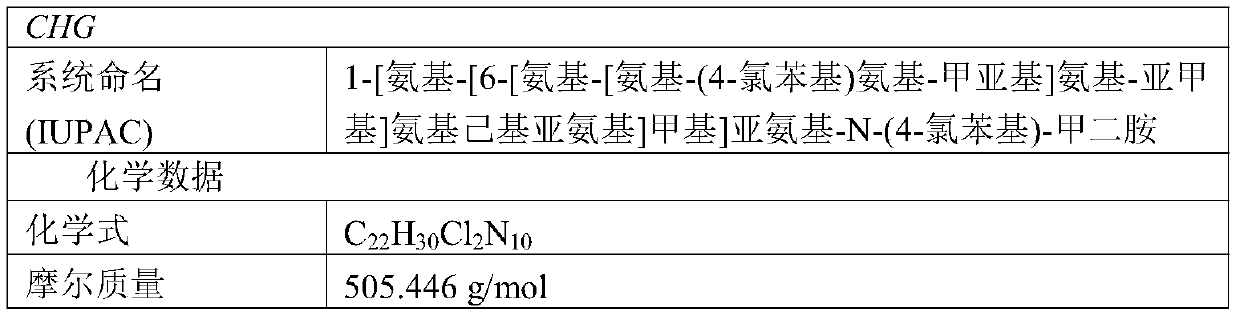
Materials and methods for the control of biofilm
A technology of biofilm and composition, applied in the direction of unknown raw materials, cosmetic preparations, medical preparations containing active ingredients, etc., can solve the problems of destroying innate immune homeostasis and failure to restore normal physiology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Example 1 - Surgical Application
[0169] In one embodiment of the invention, a sterile anti-biofilm composition is applied to a surgical site to prevent biofilm formation or to treat biofilm-associated infections at the surgical site. Surgical sites may include, for example, joint replacement, abdominal surgery, brain surgery, and oral / periodontal surgery sites.
[0170] Biofilm-associated infections that develop at surgical sites are referred to herein as "surgical site infections" or "SSIs." Surgical sites are at risk of developing SSIs due to, for example, improperly handled surgical instruments or airborne infectious agents from the operating room. SSI can be treated by administering antibiotics to the patient; however, a second surgery is usually required to treat SSI. Other procedures to treat SSI are undesirable for several reasons, eg, repeated surgical trauma to the patient, risk of recurrent infection, inadequate healing of the surgical site, and additional...
Embodiment 2
[0176] Example 2 - Intravascular Administration
[0177] In another embodiment of the invention, the anti-biofilm composition can be administered to the blood of a subject by intravascular injection.
[0178] Preferably, the injection is intravenous. The anti-biofilm composition may be a normal aqueous solution, an isotonic solution or other saline solution containing chlorhexidine.
[0179] In certain embodiments of the invention, the isotonic solution containing chlorhexidine is freshly prepared prior to administration to the subject. For example, within less than 1 minute, less than 2 minutes, about 1 minute to about 30 minutes, about 5 minutes to about 20 minutes, about 10 minutes to about 15 minutes, or any other permutation of these time periods prior to intravascular injection An isotonic solution containing the active agent is prepared.
[0180] In certain embodiments, an isotonic solution containing chlorhexidine is prepared by mixing saline solution and chlorhexid...
Embodiment 3
[0181] Example 3 - Urogenital Applications
[0182] In another embodiment of the invention, the sterile anti-biofilm composition may be administered to the urogenital tract of a subject by urogenital irrigation.
[0183] A urogenital irrigation system is a device used to irrigate one or more organs of the urogenital tract. Non-limiting examples of urogenital irrigation systems include bladder irrigation systems and urethral irrigation systems.
[0184] The sterile disinfectant solution for urogenital irrigation may be, for example, a plain aqueous solution of the active agent or an isotonic solution of the active agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com