Pyrrole sesquiterpene compound, preparation method and uses thereof
A technology for sesquiterpenes and compounds, which is applied in the field of pyrrole sesquiterpenes and their preparation, can solve the problems of low yield of chemical structures of heteroterpenes and the like, and achieve the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] In a second aspect, this embodiment provides a method for preparing the above-mentioned pyrrole sesquiterpenoids, which includes the following steps:
[0060] Step S1: fermenting and culturing Streptomyces sp.KIB 015 to obtain a culture solution;
[0061] Wherein, the Streptomyces sp.KIB 015 strain is a strain disclosed in literature (ie S.sp.KIB 015), which is: Isolation and Biosynthesis of Labdanmycins: Four New Labdane Diterpenes from Endophytic Streptomyces, Organic Chemistry Frontiers, 2018, 5 ,1272–1279.
[0062] Further, the conditions for fermenting and culturing the strains are: temperature 27-33°C, culture time 8-12 days; or temperature 29-31°C, culture time 9-11 days.
[0063] Further, the liquid medium for fermenting and cultivating the strains includes, in parts by weight: 15-25 parts of glucose, 3-8 parts of beef extract, 3-8 parts of peptone, 3-8 parts of yeast extract powder, sodium chloride 3-8 parts, calcium carbonate 1-5 parts.
[0064] Alternative...
Embodiment 1
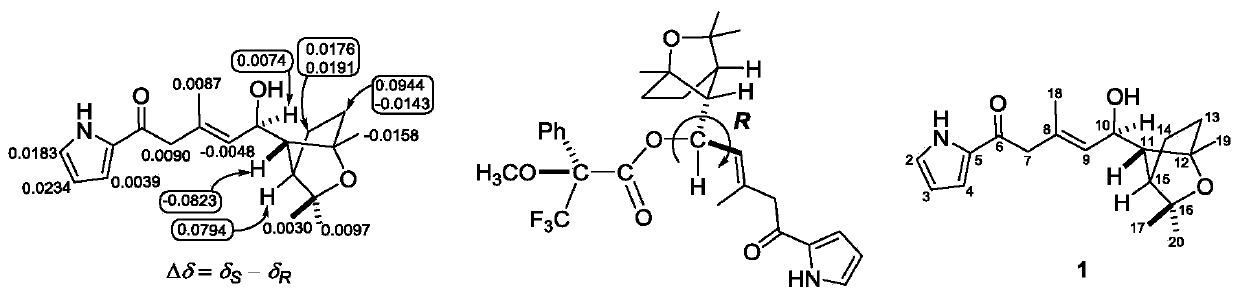
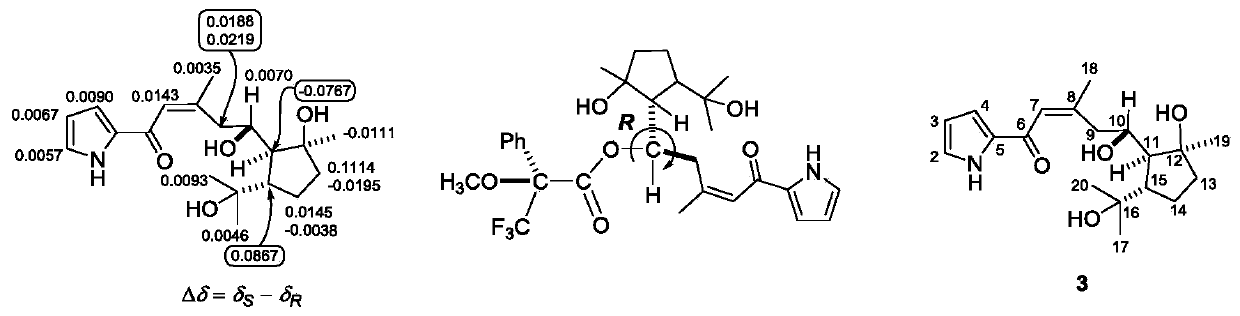
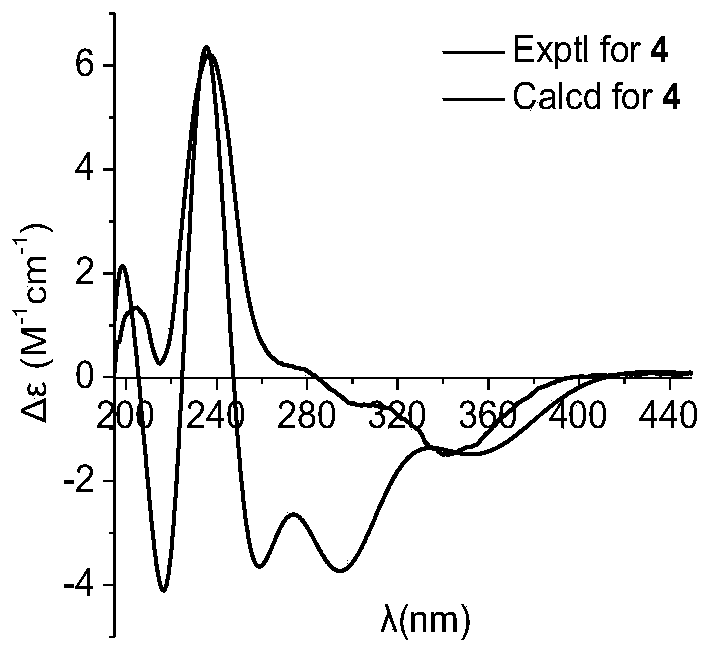
[0080] Preparation and structure identification of compound 1-6:
[0081] 1. Preparation process:
[0082] 1. Fermentation conditions
[0083] Preparation of seed culture solution: Dissolve 6.0 g of beef extract and 10.0 g of tryptone in deionized water, add deionized water to make the volume 2000 mL, and adjust the pH to 7.0. The obtained seed culture solution was evenly distributed into 40 250 mL baffled Ermlenmeyer bottles, and sterilized at 121°C for 30 minutes for future use. Streptomyces sp.KIB 015 strains were inoculated into the above-mentioned medium, and cultured on a shaker at 30° C. and 200 rpm for 48 hours to obtain a seed culture solution.
[0084] Configuration of fermentation culture medium: Dissolve 800g of glucose, 200g of beef extract, 200g of peptone, 120g of yeast extract powder, 200g of sodium chloride, and 120g of calcium carbonate in 40L of deionized water, stir to fully dissolve, and adjust the pH to 7.0. The obtained 40L fermentation culture liquid...
experiment example
[0102] The immunosuppressive effects of the six pyrrole sesquiterpenoids provided in the examples of the present invention will be evaluated in combination with cell experiments below.
[0103] 1. Experimental process:
[0104] 1. The inhibitory effect of compounds 1-6 on the proliferation of Anti-human-CD3 / CD28-induced activation and phytohemagglutinin-induced activation of human T cells.
[0105] The inhibitory effects of compounds 1-6 on the proliferation of Anti-human-CD3 / CD28-induced activation and phytohemagglutinin-induced activation of human T cells were determined by flow cytometry analysis, and 5-carboxyfluorescein diacetatesuccinimide ester (CFSE) was selected as Marker, the situation that the T cells that are not induced and activated and the corresponding compound are treated as a negative control (0%), the situation that the T cells that are induced and activated are not treated by the corresponding compound is used as a positive control (100%), and the results a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com