EPCs lysate-sensitized DC vaccine derived from tumor microenvironment and preparation method thereof
A tumor microenvironment and lysate technology is applied in the field of DC vaccine sensitized by EPCs lysate derived from tumor microenvironment and its preparation field, which can solve the problems of unsatisfactory effect and other problems, and achieve the enhancement of anti-tumor angiogenesis and inhibition of tumor blood vessels. generated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
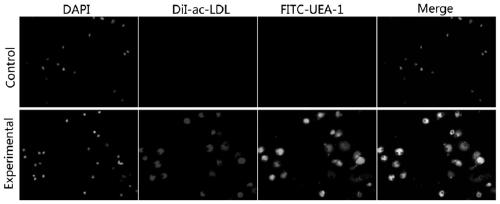
Image
Examples
Embodiment 1
[0038] 1. Isolation of bone marrow mononuclear cells and culture of endothelial progenitor cells (EPCs)
[0039] 1.2.2.1 Isolation of mouse bone marrow mononuclear cells and culture of endothelial progenitor cells (EPC)
[0040] 1.2.2.1.1 Isolation of mouse bone marrow mononuclear cells
[0041] 1) C57BL / 6 mice were sacrificed (collecting blood for later use),
[0042] 2) Aseptically take the tibia and femur, and use serum-free medium (DMEM) to quickly wash the bone marrow into a centrifuge tube until the washing solution is clear.
[0043] 3) Repeatedly blow and beat the washing solution, and then add it slowly into the test tube containing the lymphocyte separation solution according to the ratio of 1:1 along the tube wall with a dropper.
[0044] 4) Centrifuge at 2000r / mi for 20min at 20°C,
[0045] 5) The gray-white cloud layer cells between the lymphocyte separation liquid and the culture medium obtained by centrifugal separation are bone marrow mononuclear cells.
[...
Embodiment 2
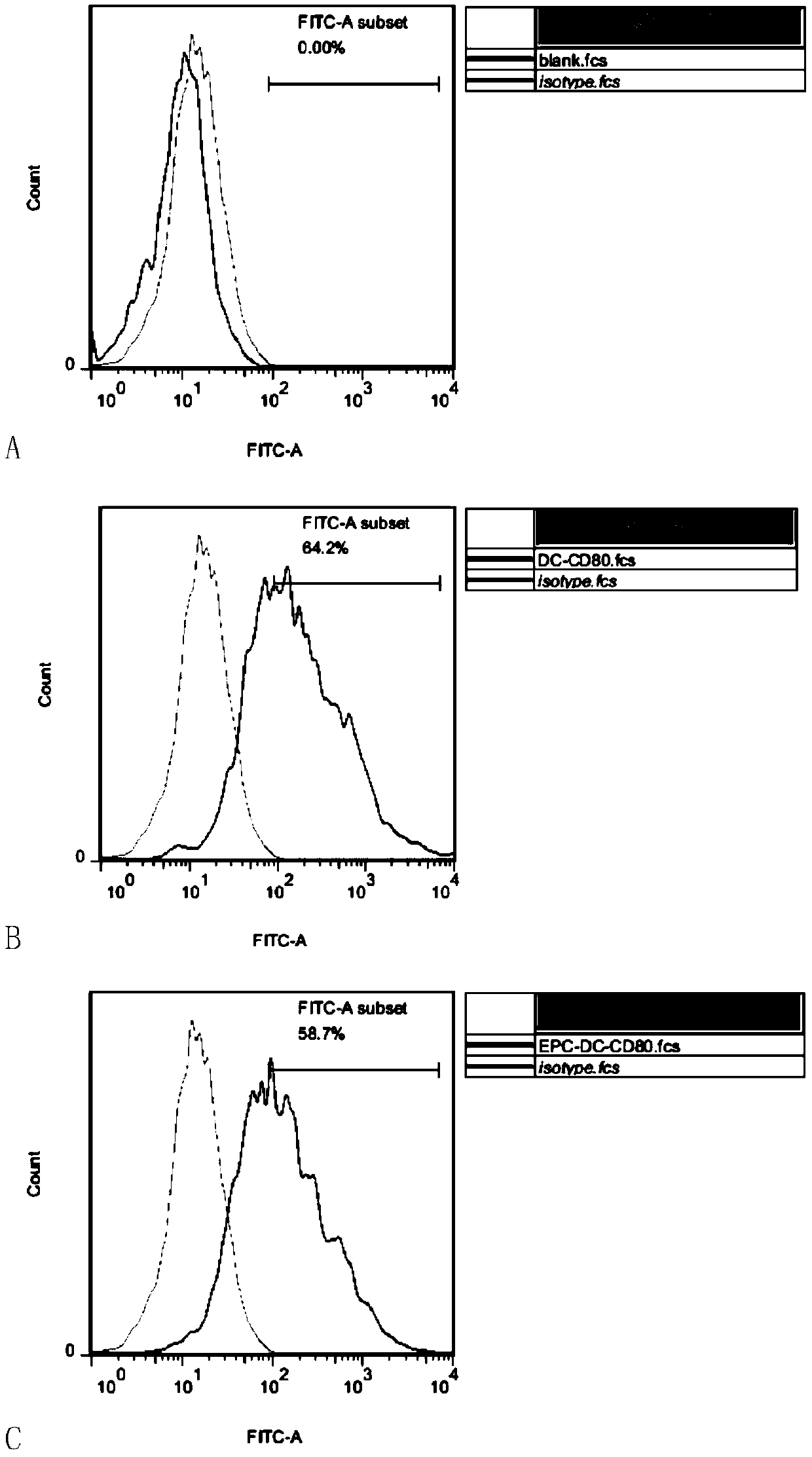
[0155] Example 2: Expression of CD80, CD86, and MHCII in DC cells sensitized by LLC-EPCs
[0156] experimental method:
[0157] The aforementioned cells (mature DC cells obtained in Example 1) were digested with trypsin and washed once with PBS. Block with PBS containing 5% FBS or 5% BSA for 30 minutes on ice. Wash once with PBS, resuspend with PBS containing 0.5% BSA, and adjust the cell density to 2×106 cells / mL. Take 100 μL of cells for staining, add isotype control and anti-CD86, CD80, MHC-II antibodies respectively according to the dilution ratio in the instructions. Incubate at 4 degrees for 30 minutes. Immature dendritic cells were used as negative control. Centrifuge and remove supernatant. Wash once with PBS. Add 400 μL PBS to resuspend, flow on the machine.
[0158] Solution preparation:
[0159] 5%BSA:2.5gBSA+50mLPBS
[0160] 0.5%BSA:0.25gBSA+50mLPBS
[0161] Experimental results:
[0162] see Figure 3-5 , the results showed that the expressions of CD80...
Embodiment 3
[0163] Example 3: Effect of LLC-EPC-DC on Proliferation of Mixed Lymphocytes
[0164] experimental method:
[0165] Isolation of T lymphocytes:
[0166] 1) Take the spleen of the mouse under aseptic conditions, and grind it through a 40um filter;
[0167] 2) Collect the cell suspension and add 3ml of erythrocyte lysate;
[0168] 3) Wash cells twice with PBS, 1200rpm, 5min, resuspend and count;
[0169] 4) Take 1x107 cells and add 40ul buffer;
[0170] 5) Add 5ul Biotin-Antibody Cocktail, 4°C for 5min;
[0171] 6) Add 30ul buffer, add 10ul Anti-Biotin Microbeads, and treat at 4°C for 5min;
[0172] 7) Centrifuge at 1200rpm for 5min, add 500ul buffer to pass through the column;
[0173] 8) Collect the cells and count under the counting plate;
[0174] Buffer: PBS containing 0.5% BSA, 2mM EDTA.
[0175] Mixed lymphocyte reaction and CCK-8 proliferation detection:
[0176] 1) Collect the prepared DC cells, add 25mg / L mitomycin, and treat at 37°C for 45min
[0177]2) Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com