Synthesis method of dolutegravir key intermediate
A technology of dolutegravir and a synthesis method, which is applied in the field of dolutegravir compound synthesis, can solve the problems of unfavorable industrialized production, high price of raw materials and high production cost, and achieves the effects of low cost, good product quality and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
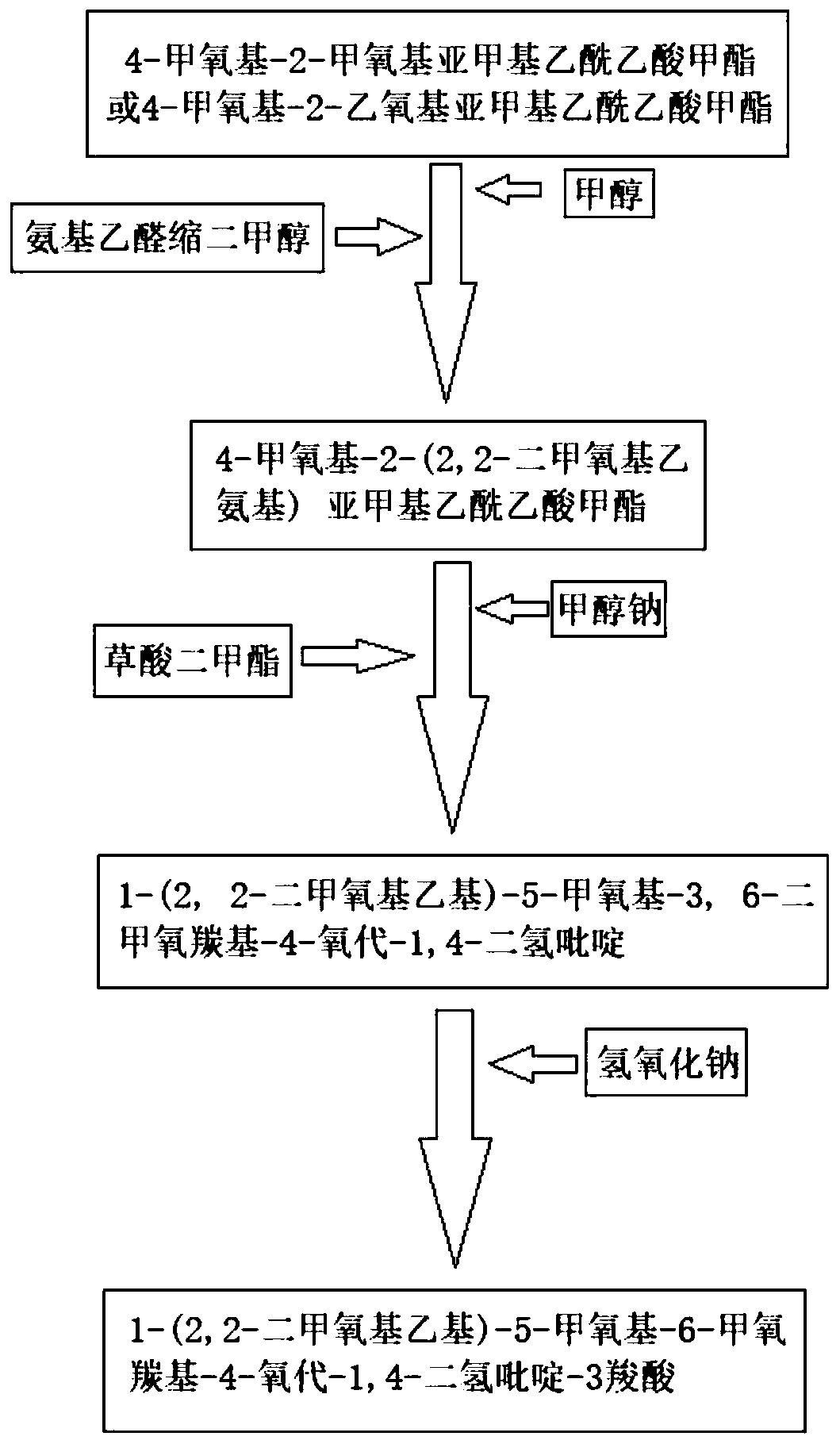
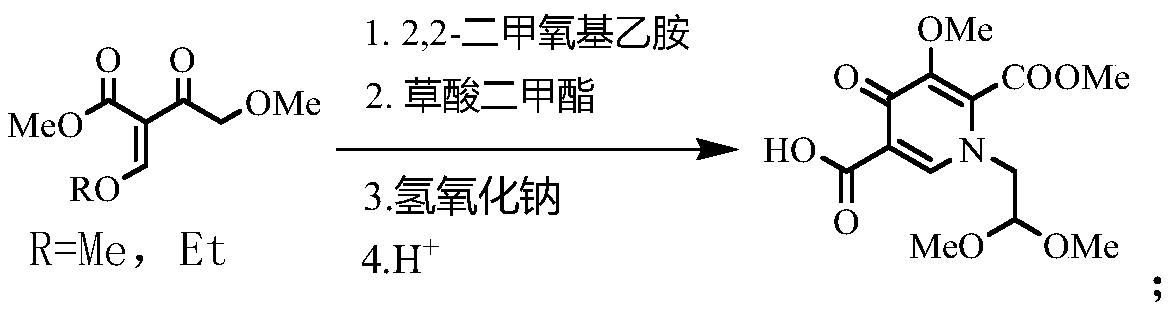
[0027] The specific synthetic method of compound 1-(2,2-dimethoxyethyl)-5-methoxy-6-methoxycarbonyl-4-oxo-1,4-dihydropyridine-3-carboxylic acid:
[0028] Add 18.8g of methyl 4-methoxy-2-methoxymethyleneacetoacetate and 100g of methanol into a 500mL four-neck flask, stir, add 10.1g of aminoacetaldehyde dimethyl acetal dropwise at room temperature, and then React for 2 hours. After the reaction is completed, add 6.48g of sodium methoxide, then raise the temperature to 30-35°C, add 13g of dimethyl oxalate in batches, and stir for another 5 hours after the addition is completed. After the reaction is completed, cool down to 0-5°C. ℃, add 44g of 10% sodium hydroxide solution, after the reaction is completed, adjust pH=3~3.5, precipitate solid, stir and crystallize for 3 hours, add appropriate amount of water, filter, and obtain 28.8g of the target product after drying, yield 91.4%, content 99.51%.
specific Embodiment 2
[0030] The specific synthetic method of compound 1-(2,2-dimethoxyethyl)-5-methoxy-6-methoxycarbonyl-4-oxo-1,4-dihydropyridine-3-carboxylic acid:
[0031] Add 20.2 g of methyl 4-methoxy-2-ethoxymethylene acetoacetate and 100 g of methanol into a 500 mL four-neck flask, stir, add 10.1 g of aminoacetaldehyde dimethyl acetal dropwise at room temperature, and then React for 2 hours. After the reaction is completed, add 6.48g of sodium methoxide, then raise the temperature to 30-35°C, add 13g of dimethyl oxalate in batches, and stir for another 5 hours after the addition is completed. After the reaction is completed, cool down to 0-5°C. ℃, add 44g of 10% sodium hydroxide solution, after the reaction is completed, adjust the pH=3 to 3.5, precipitate the solid, stir and crystallize for 3 hours, add an appropriate amount of water, filter, and dry to obtain the target product 28.6g, the yield 90.8%, content 99.51%.
specific Embodiment 3
[0033] The specific synthetic method of compound 1-(2,2-dimethoxyethyl)-5-methoxy-6-methoxycarbonyl-4-oxo-1,4-dihydropyridine-3-carboxylic acid:
[0034] Add 18.8g of methyl 4-methoxy-2-methoxymethyleneacetoacetate and 100g of methanol into a 500mL four-neck flask, stir, add 10.1g of aminoacetaldehyde dimethyl acetal dropwise at room temperature, and then React for 2 hours. After the reaction is completed, add 6.48g of sodium methoxide, then raise the temperature to 60-65°C, add 13g of dimethyl oxalate in batches, and stir for another 5 hours after the addition is completed. After the reaction is completed, cool down to 0-5°C. ℃, add 44g of 10% sodium hydroxide solution, after the completion of hydrolysis, adjust the pH=3~3.5, precipitate solid, continue to stir and crystallize for 3 hours, then add an appropriate amount of water, filter, and obtain 26.8g of the target product after drying. The rate is 85.2%, and the content is 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com