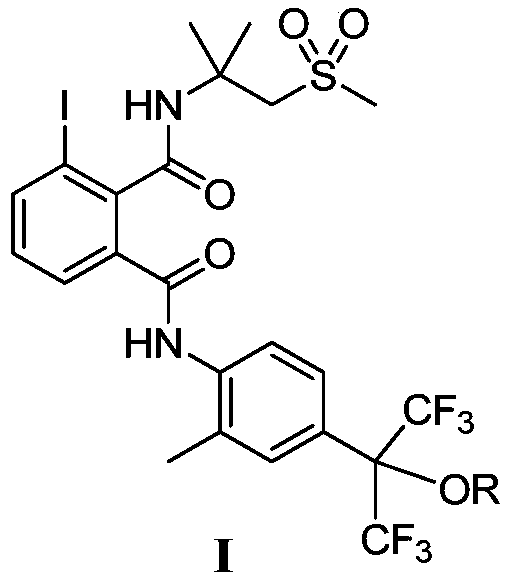
Alkoxy hexafluoroisopropyl-containing phthalic diamide compounds and application thereof
A technology containing alkoxyhexafluoroisopropyl and alkoxyhexafluoroisopropyl, which is applied in the field of organic synthesis and preparation, can solve the problems of high synthesis cost, high price, and reduced fat solubility of compounds, and achieve strong insecticide The effect of activity, low cost and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
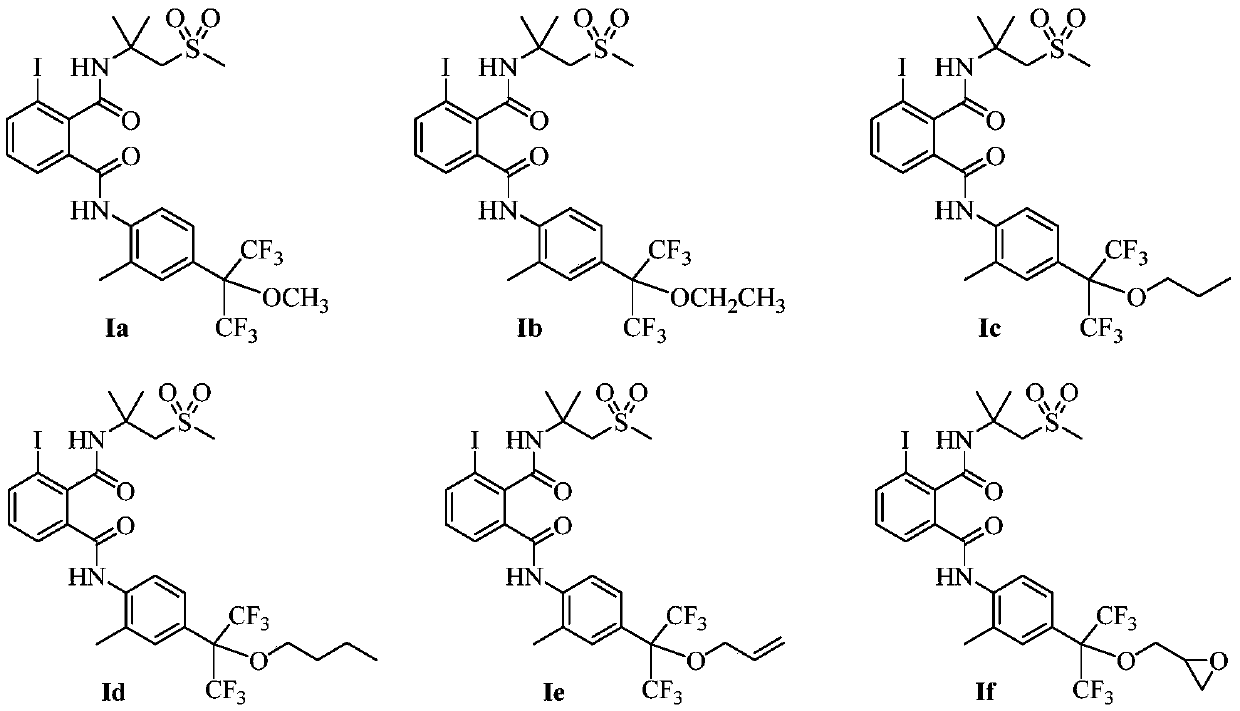
[0035] 3-iodo-N'-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-N-{2-methyl-4-[2,2,2-trifluoro-1-methyl Synthesis of oxy-1-(trifluoromethyl)ethyl]phenyl}phthalamide (a compound represented by formula Ia, abbreviated as compound Ia, the same below):
[0036]
[0037] Hexafluoroacetone trihydrate (4.84 g) and p-toluenesulfonic acid (0.2 g) were dissolved in 10 ml of xylene, and o-methylaniline (2.14 g) was added dropwise at 90°C. The reaction solution was stirred for 12 hours at a temperature of 130°C, and the reaction was followed by TLC. After the reaction, the reaction solution was cooled to room temperature, the crystals were filtered, and the filter cake was washed with petroleum ether to obtain 2-(3'-methyl-4'-aminophenyl)-1,1,1,3,3, The 3-hexafluoro-2-propanol compound represented by formula V is about 5.16 g, and the yield is 95%.
[0038] Add 2.73 grams of 2-(3'-methyl-4'-aminophenyl)-1,1,1,3,3,3-hexafluoro-2-propanol compound represented by formula V and 4.89 grams of cesium carb...
Embodiment 2
[0046] 3-iodo-N'-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-N-{2-methyl-4-[2,2,2-trifluoro-1-ethane Synthesis of oxy-1-(trifluoromethyl)ethyl]phenyl}phthalamide (compound Ib):
[0047]
[0048] The preparation method was the same as that in Example 1. In the synthesis of VIb, ethyl iodide was used to replace methyl iodide in Example 1 to obtain a white solid (Compound Ib). The total yield of the three-step reaction was 63%, and the melting point was 136.7-138.9°C.
[0049] 1 H NMR(400MHz, DMSO-d 6 )δ=9.73(s,1H),8.43(s,1H),8.03(d,J=8.0Hz,1H),7.84(d,J=8.0Hz,1H),7.74(d,J=7.2Hz, 1H),7.46–7.44(m,2H),7.29(t,J=8.0Hz,1H),3.68(s,2H),3.63(q,J=6.8Hz,2H),2.95(s,3H), 2.38(s,3H),1.57(s,6H),1.32(t,J=6.8Hz,3H)ppm;
[0050] 13 C NMR(100MHz, DMSO-d 6 )δ=167.6, 165.5, 141.2, 140.7, 138.3, 136.0, 132.3, 130.1, 129.4, 127.3, 125.6, 124.7, 123.7, 123.6, 95.3, 82.1, 62.3, 60.7, 52.4, 43.0, 26.1, 18.1, 14.9ppm;
[0051] 19 F NMR(376MHz, DMSO-d 6 )δ=–70.4(s,6F)ppm.
[0052] HRMS(ESI): C 25 H 27 F 6 IN 2 O 5 SNa[...
Embodiment 3
[0054] 3-iodo-N'-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-N-{2-methyl-4-[2,2,2-trifluoro-1-normal Synthesis of propoxy-1-(trifluoromethyl)ethyl]phenyl}phthalamide (compound Ic):
[0055]
[0056] The preparation method is the same as that in Example 1. In the synthesis of VIc, 1-bromopropane was used to replace methyl iodide in Example 1 to obtain a white solid (Compound Ic). The total yield of the three-step reaction was 61%, and the melting point was 142.1-145.0°C.
[0057] 1 H NMR(400MHz, DMSO-d 6 )δ=9.72(s,1H), 8.41(s,1H), 8.02(d,J=7.6Hz,1H), 7.81(d,J=8.0Hz,1H), 7.72(d,J=7.6Hz, 1H),7.44–7.42(m,2H),7.28(t,J=7.6Hz,1H), 3.65(s,2H), 3.51(t,J=6.4Hz,2H), 2.94(s,3H), 2.36(s,3H),1.73-1.68(m,2H),1.54(s,6H),0.95(t,J=7.6Hz,3H)ppm;
[0058] 13 C NMR(101MHz, DMSO-d 6 )δ=167.7,165.5,141.3,140.7,138.3,136.1,132.4,130.1,129.5,127.3,125.7,124.8,123.7,123.5,95.4,82.1,67.7,60.8,52.4,43.1,26.1,22.4,18.1,10.0 ppm;
[0059] 19 F NMR(376MHz, DMSO-d 6 )δ=–70.3(s,6F)ppm.
[0060] HRMS(ESI): C 26 H 29 F 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com