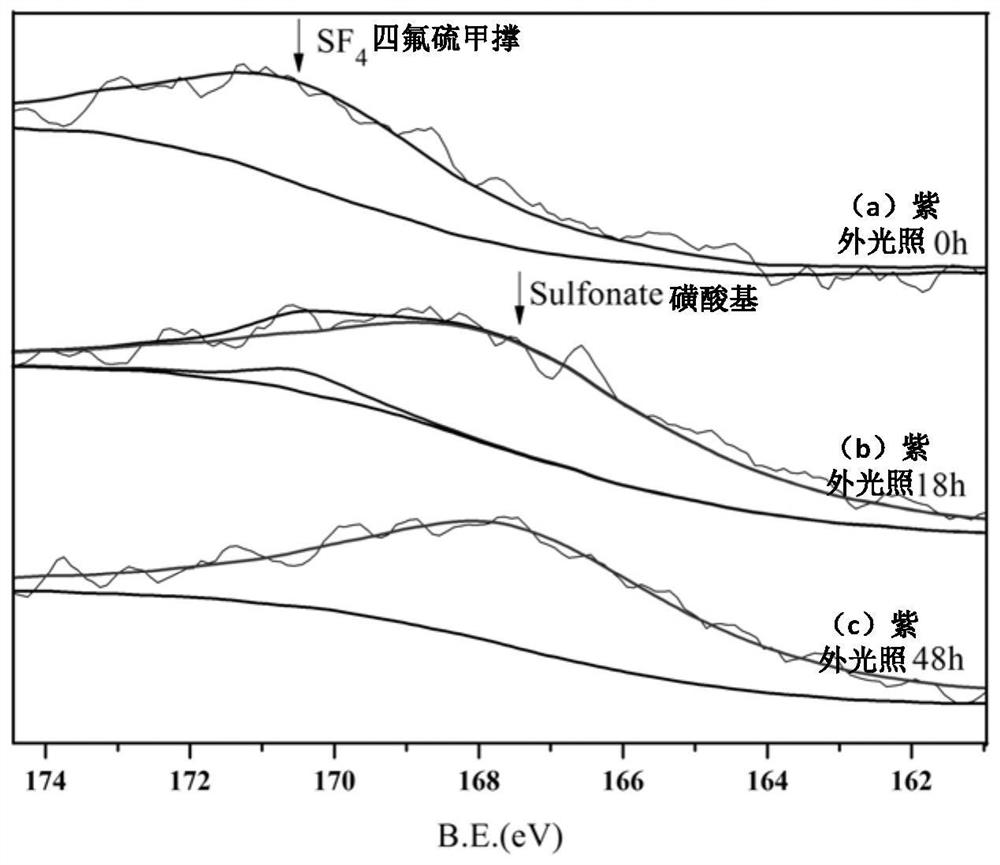
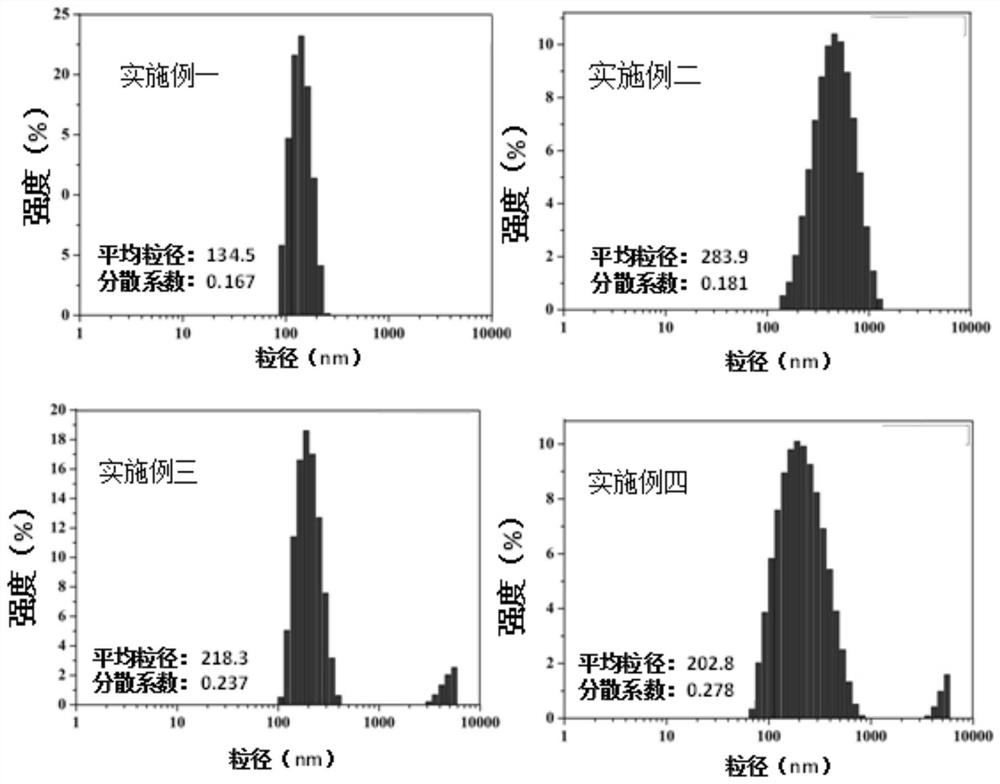
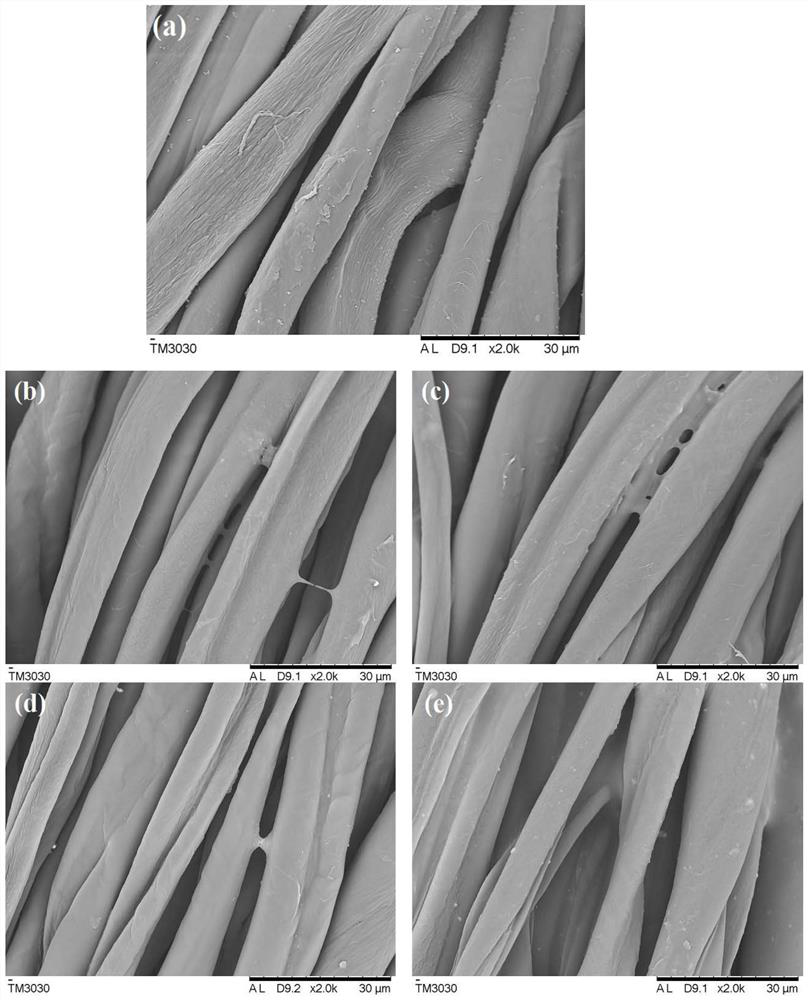
Perfluoroalkyltetrafluorosulfurmethylene styrene copolymer and its application
A technology of perfluoroalkyl tetrafluorothiomethyl and perfluoroalkyl thiohalobenzene is applied in the field of textile finishing auxiliaries and the preparation of fluorine-containing materials to achieve the effects of strong activity, improved liquid repellency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In this embodiment, nonafluorobutyl tetrafluoromethylene styrene copolymer type finishing agent is synthesized, and the specific steps are as follows:
[0058] (1) Synthesis of 4-(nonafluorobutyltetrafluorosulfurmethylene)bromobenzene
[0059] In a 250 ml three-neck flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, add 5.1 g of p-bromothiophenol, 10.4 g of nonafluoro-1-iodobutane, 0.5 g of copper acetate and 40 g of 1,4-dioxane, stirred and heated to 70°C, the reaction solution turned yellow. Dissolve 7.2 g of benzoyl peroxide in 45 g of 1,4-dioxane, slowly add it dropwise through a constant pressure dropping funnel, and keep it warm for 4 h after the drop is complete. After the reaction was completed, 1,4-dioxane was removed by rotary evaporation under reduced pressure, the resulting precipitate was filtered off, washed with water, dried over anhydrous magnesium sulfate for 8 h, and then rotary evaporat...
Embodiment 2
[0076] In this example, a 4-(tridecafluorohexyltetrafluoromethylene) styrene copolymer type finishing agent was synthesized, and the specific steps were as follows:
[0077] (1) Synthesis of 4-(tridecafluorohexyltetrafluorosulfurmethylene) bromobenzene
[0078] Referring to the synthesis steps of 4-(nonafluorobutylthio)bromobenzene in Example 1, first synthesize 4-(tridecafluorohexylthio)bromobenzene. Add 13.4 g of perfluoroiodohexane when feeding, and use the same amount of other materials to obtain 8.5 g of the product 4-(tridecafluorohexylthio)bromobenzene, yield: 62.0%. product 1 H NMR (400 MHz, CDCl 3 ): δ 7.41 (d, J = 8.4 Hz, 2H, o -H), 7.32 (d, J = 8.4 Hz, 2H, m -H). 19 F NMR (564 MHz, CDCl 3 ): δ -80.78 (3F, CF 3 CF 2 CF 2 CF 2 CF 2 CF 2 S), -86.88 (2F, CF 3 CF 2 CF 2 CF 2 CF 2 CF 2 S), -119.30 (2F, CF 3 CF 2 CF 2 CF 2 CF 2 CF 2 S), -121.54 (2F, CF 3 CF 2 CF 2 CF 2 CF 2 CF 2 S), -122.86 (2F, CF 3 CF 2 CF 2 CF 2 CF 2 CF ...
Embodiment 3
[0094] In this example, a 4-(tridecafluorohexyltetrafluoromethylene) styrene copolymer type finishing agent was synthesized, and the specific steps were as follows:
[0095] (1) Synthesis of 4-(tridecafluorohexyltetrafluorosulfurmethylene) chlorobenzene
[0096] The synthesis steps of 4-(tridecafluorohexyltetrafluoromethylene) chlorobenzene refer to the synthesis steps of 4-(nonafluorobutyltetrafluoromethylene) bromobenzene in Example 1, and the reactants and dosage are: 4 -3.0 g of (tridecafluorohexylthio)chlorobenzene, 5.8 g of potassium fluoride, and 1.0 g of chlorine gas gave 2.8 g of the product, yield: 78.2%.
[0097] (2) Synthesis of 4-(tridecafluorohexyltetrafluorosulfurmethylene)styrene
[0098] The synthesis of 4-(tridecafluorohexyltetrafluoromethylene)styrene refers to the synthesis steps of 4-(nonafluorobutyltetrafluoromethylene)styrene in Example 1. The reactants and dosage are intermediate 4 -(Tridecafluorohexyltetrafluorosulfurmethylene)chlorobenzene 1.3 g, Pd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com