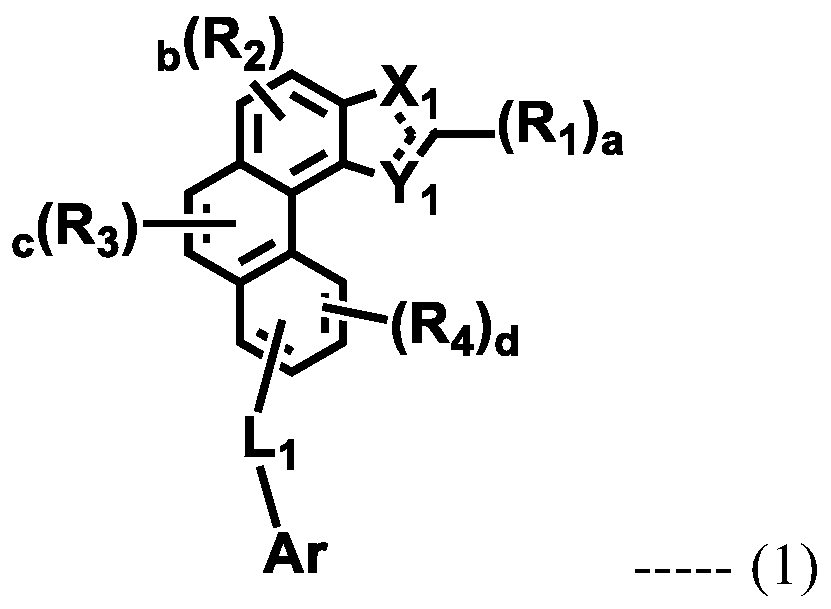
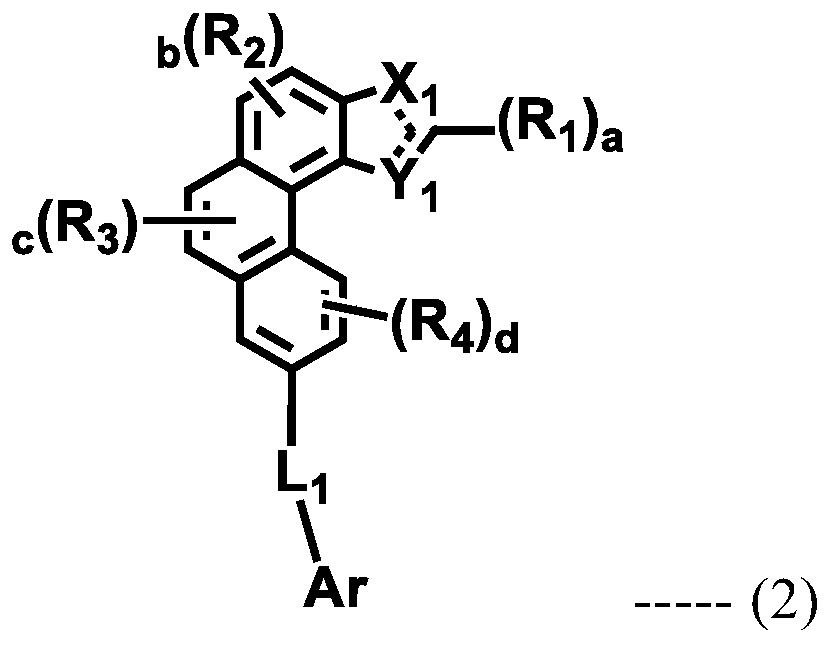
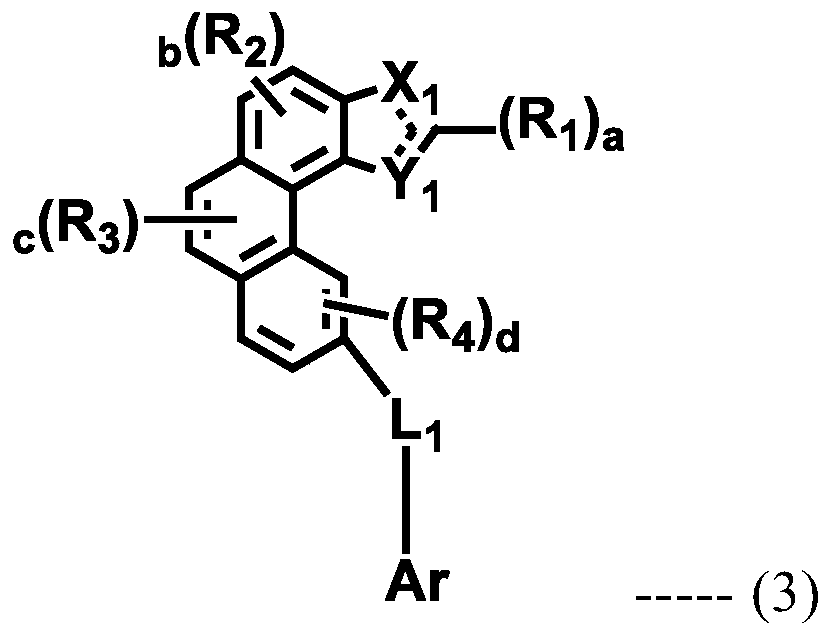
Organic electroluminescent compound and organic electroluminescent device comprising the same
An electroluminescence and compound technology, applied in the field of organic electroluminescence compounds, can solve the problems of lowering the color purity, and achieve the effects of improving the luminous efficiency, improving the lifespan and prolonging the lifespan.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0084] Example 1: Preparation of Compound C-23
[0085]
[0086] Compound A (3.5g, 8mmol), 3-(3-bromophenyl) fluoranthene (2.5g, 7mmol), tetrakis (triphenylphosphine) palladium (0.4g, 0.4mmol), sodium carbonate (2.5g, 18 mmol), 36 mL of toluene, 9 mL of ethanol, and 9 mL of distilled water were added to the reactor, and stirred at 120° C. for 3 hours. After the reaction was completed, the organic layer mixture was washed with distilled water and extracted with ethyl acetate. The extracted organic layer was dried with magnesium sulfate, and the solvent was removed by a rotary evaporator. Thereafter, the residue was purified by column chromatography to obtain compound C-23 (3.4 g, yield: 85%).
[0087] MW UV PL melting point C-23 571.68 390nm 457nm 209℃
example 2
[0088] Example 2: Preparation of Compound C-24
[0089]
[0090] Compound A (3.3g, 8mmol), 2-(3-bromophenyl)triphenylene (3g, 8mmol), tetrakis(triphenylphosphine)palladium (0.3g, 0.3mmol), potassium carbonate (2.7g, 20mmol ), 40 mL of toluene, 10 mL of ethanol, and 10 mL of distilled water were added to the reactor, and stirred at 120° C. for 3 hours. After the reaction was completed, the organic layer mixture was washed with distilled water and extracted with ethyl acetate. The extracted organic layer was dried with magnesium sulfate, and the solvent was removed by a rotary evaporator. Thereafter, the residue was purified by column chromatography to obtain compound C-24 (2.5 g, yield: 53%).
[0091] MW UV PL melting point C-24 597.72 395nm 481nm 287℃
example 3
[0092] Example 3: Preparation of Compound 1-4
[0093]
[0094] 1) Preparation of compound 1-1
[0095] Compound B (CAS: 1044146-16-8, 36g, 124mmol), 4-chloro-2-formylphenylboronic acid (25.2g, 136mmol), tetrakis (triphenylphosphine) palladium (5.7g, 5.0mmol), Sodium carbonate (33 g, 150 mmol), 600 mL of toluene, 150 mL of ethanol, and 150 mL of distilled water were added to the reactor and stirred at 140° C. for 3 hours. After the reaction was completed, the precipitated solid was washed with distilled water and methanol. The obtained compound 1-1 was used in the next reaction without further purification.
[0096] 2) Preparation of compound 1-2
[0097] Compound 1-1 (45.6 g, 130 mmol), (methoxymethyl)triphenylphosphine chloride (74.3 g, 217 mmol), and 1,500 mL of tetrahydrofuran were added to the reactor, and the reaction mixture was stirred for 5 minutes. Then, potassium tert-butoxide (1M in THF, 220 mL) was slowly added dropwise at 0°C. The temperature was raised...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com