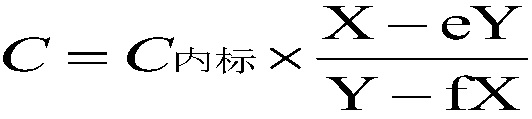Method for rapidly detecting small molecule compound
A technology for small molecule compounds and target compounds, applied in the field of analytical chemistry, can solve problems such as poor universality, cumbersome derivatization steps, and small application range, and achieve the effect of avoiding interference, avoiding no response or low response value, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
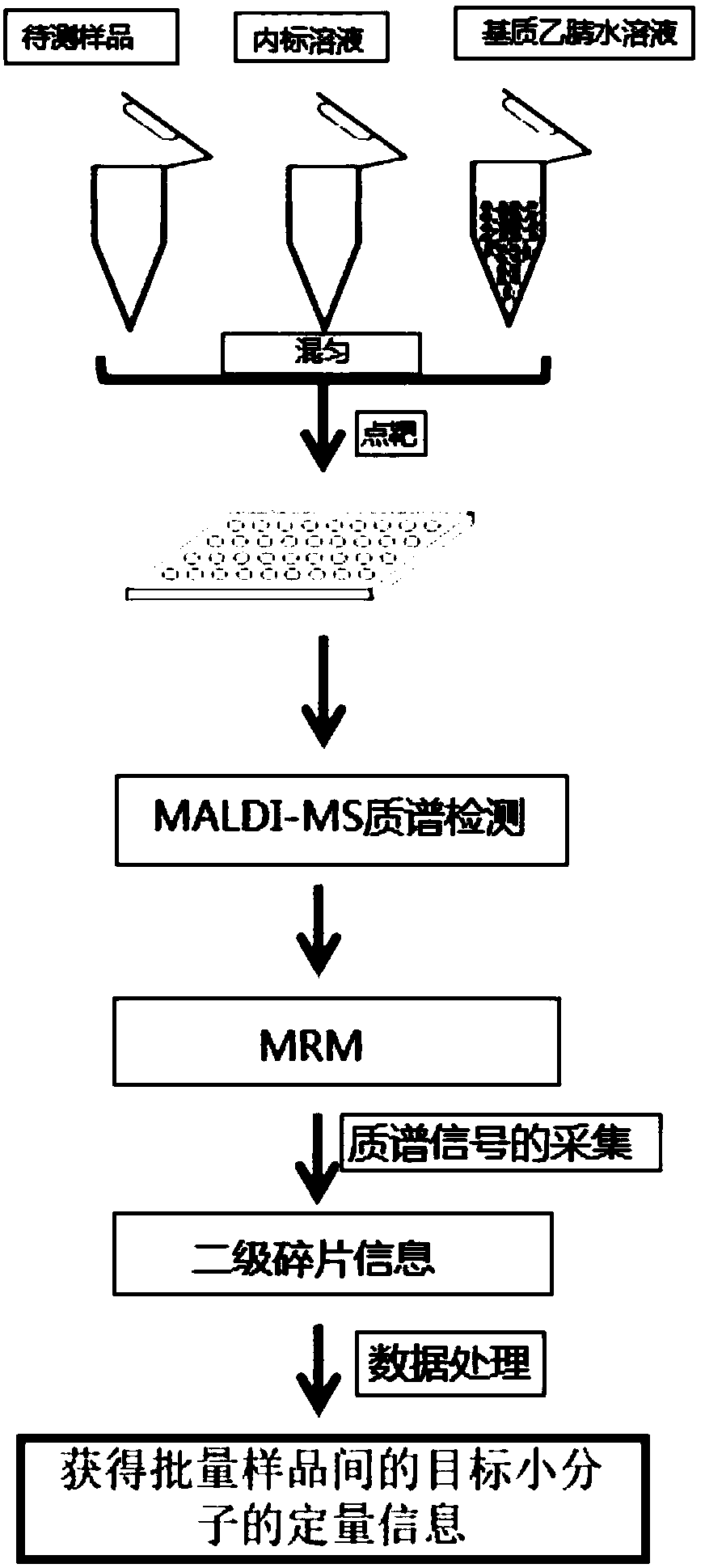
[0057] Such as figure 1 As shown, a rapid detection of citrate-2,4-C 2 method, including the following steps:
[0058] (1) Selected citric acid-2,4-C 2 for the target compound;
[0059] (2) 1,5-diaminonaphthalene as a matrix is formulated into a 50% acetonitrile aqueous solution with a concentration of 10 mg / mL to obtain a matrix acetonitrile aqueous solution;
[0060] (3) Citric acid-2,4- 13 C 2 Prepare a 50% acetonitrile aqueous solution with a concentration of 10 mg / mL to obtain an internal standard solution;
[0061] (4) Mix the acetonitrile aqueous solution of the matrix, the internal standard solution and the sample to be tested at a volume ratio of 10:1:1, and repeatedly blow or vortex to mix to obtain a sample injection mixture;
[0062] (5) Manually point each 1 ul of the injection mixture onto a sample target of the MALDI-MS to form a sample point and dry it;
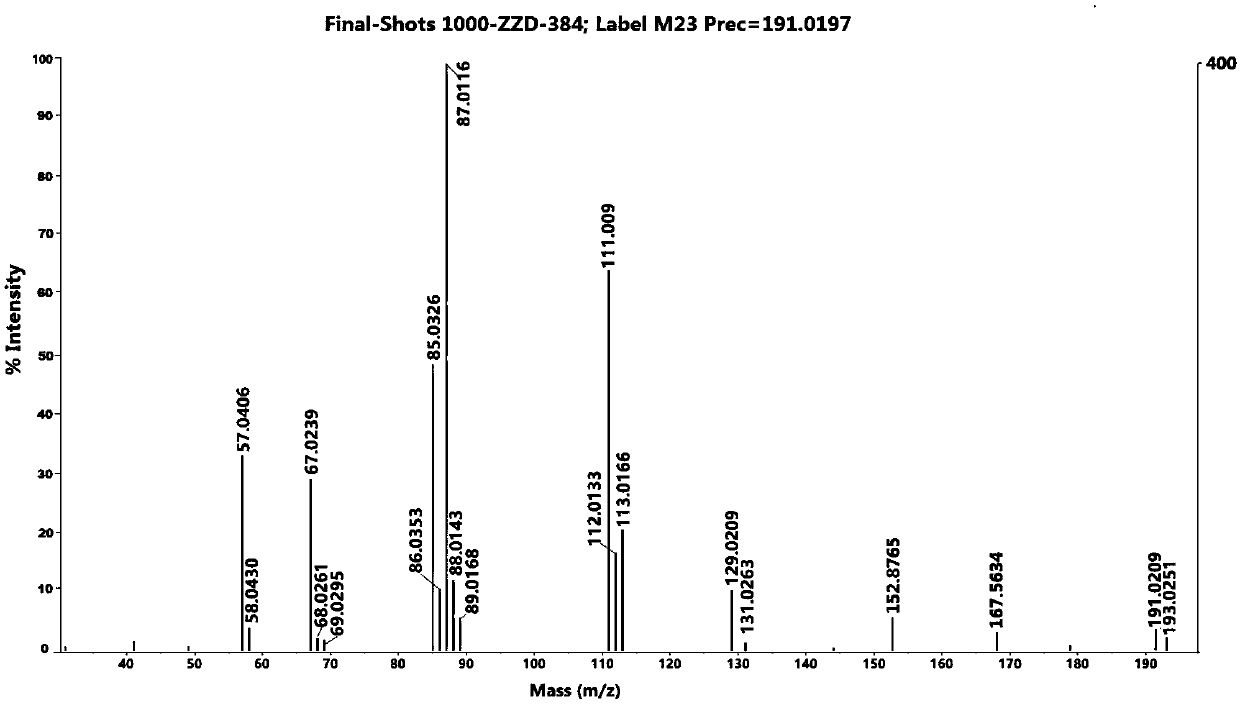
[0063] (6) Put the dried sample target into the mass spectrometer sample chamber, enter the target...
Embodiment 2
[0073] Such as figure 1 As shown, a method for rapidly detecting malic acid-2-C, glucose-1-C, glutamic acid-5-C, glucose-6-phosphate-1-C, comprising the following steps:
[0074] (1) select malic acid-2-C, glucose-1-C, glutamic acid-5-C, glucose-6-phosphate-1-C as target compounds;
[0075] (2) 1,5-diaminonaphthalene as a matrix is formulated into a 50% acetonitrile aqueous solution with a concentration of 10 mg / mL to obtain a matrix acetonitrile aqueous solution;
[0076] (3) Malic acid-2- purchased from sigma company 13 C. Glucose-1- 13 C. Glutamic acid-5- 13 C. Glucose-6-phosphate-1- 13 C is prepared into a 50% acetonitrile aqueous solution with a concentration of 10 mg / mL to obtain a mixed and stable internal standard solution;
[0077](4) Mix the acetonitrile aqueous solution of the matrix, the internal standard solution and the sample to be tested at a volume ratio of 10:1:1, and repeatedly blow or vortex to mix to obtain a sample injection mixture;
[0078] (5) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| laser intensity | aaaaa | aaaaa |
| laser intensity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com