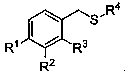
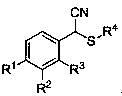
Alpha-thioether aryl acetonitrile compound and synthesis method thereof
An arylacetonitrile and compound technology, applied in the field of α-thioether arylacetonitrile compounds and their synthesis, can solve the problems of harsh reaction conditions, expensive raw material preparation, limited product types, etc., and achieves easy availability of raw materials and good application prospects , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: 2-(tert-butylthio)-2-phenylacetonitrile
[0041] 2-(tert-butylthio)-2-phenylacetonitrile adopts the following steps: 1. add 5.4 grams of benzyl sulfide (30 mmol) in a 500 milliliter three-necked flask, 6.1 milliliters of tert-butylisonitrile (54 mmol), 0.78 grams of three Silver fluoromethanesulfonate (3 mmol), 13.60 g of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (60 mmol), 300 ml of toluene, heated to 100°C. Track the reaction with thin-layer chromatography, and react until the raw materials disappear after 3 hours; ② The crude product obtained after removing the solvent from the reaction system is directly separated and purified by column chromatography (developing solvent: petroleum ether: ethyl acetate=100:1), Obtain 4.3 grams of 2-(tert-butylthio)-2-phenylacetonitrile, whose structural formula is: The yield was 70%. This compound belongs to the first reported synthesis, and the synthesis method has mild reaction conditions, good yield and good applica...
Embodiment 2
[0047] Example 2: 2-[(1,1'-biphenyl)-4-yl]-2-(tert-butylthio)acetonitrile
[0048] 2-[(1,1'-biphenyl)-4-yl]-2-(tert-butylthio)acetonitrile adopts the following steps: 1. Add 7.69 grams of ([1,1'- Biphenyl]-4-ylmethyl)(tert-butyl)sulfide (30mmol), 6.8ml tert-butylisonitrile (60mmol), 1.17g silver triflate (4.5mmol), 27.20g 2,3-bis Chloro-5,6-dicyano-1,4-benzoquinone (120mmol), 300ml of toluene, heated to 100°C. Track the reaction with thin-layer chromatography, and react until the raw materials disappear after 3 hours; ② The crude product obtained after removing the solvent from the reaction system is directly separated and purified by column chromatography (developing solvent: petroleum ether: ethyl acetate=100:1), Obtain 2.83 grams of 2-[(1,1'-biphenyl)-4-yl]-2-(tert-butylthio)acetonitrile, whose structural formula is: The yield was 34%. This compound belongs to the first reported synthesis, and the synthesis method has mild reaction conditions, good yield and good applic...
Embodiment 3
[0055] Example three: 2-(tert-butylthio)-2-(4-isopropylphenyl)acetonitrile
[0056] 2-(tert-butylthio)-2-(4-isopropylphenyl) acetonitrile adopts the following steps: 1. add 6.67 grams of tert-butyl (4-isopropylbenzyl) sulfide in a 500 ml three-necked flask Ether (30 mmol), 6.1 mL tert-butylisonitrile (54 mmol), 0.78 g silver triflate (3 mmol), 13.60 g 2,3-dichloro-5,6-dicyano-1,4-benzoquinone ( 60mmol), 300ml of toluene, heated to 100°C. Track the reaction with thin-layer chromatography, and react until the raw materials disappear after 3 hours; ② The crude product obtained after removing the solvent from the reaction system is directly separated and purified by column chromatography (developing solvent: petroleum ether: ethyl acetate=100:1), Obtain 4.59 grams of 2-(tert-butylthio)-2-(4-isopropylphenyl)acetonitrile, whose structural formula is: The yield was 62%. This compound belongs to the first reported synthesis, and the synthesis method has mild reaction conditions, g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com