Method for producing urolithins
A manufacturing method and a technology for urolithin, which are applied in the directions of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problem of no report, no report producing urolithin A, and inability to produce ellagic acid metabolite urolith. A and other issues, to achieve the effect of promoting autophagy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0229] Hereinafter, the present invention will be described in further detail based on specific examples, but the present invention is not limited to these examples.
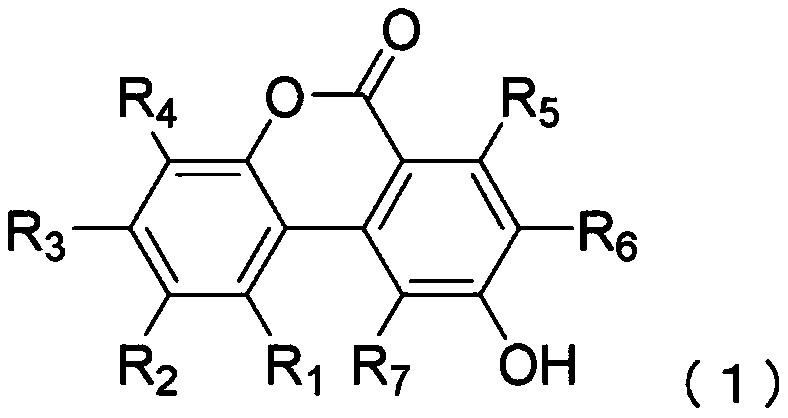
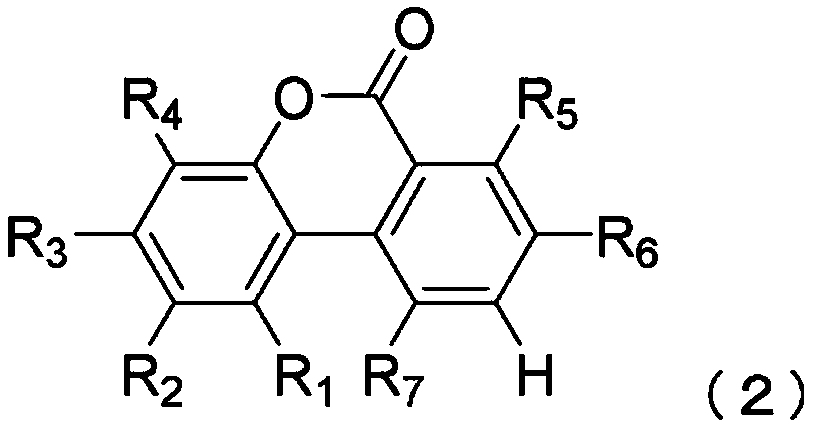
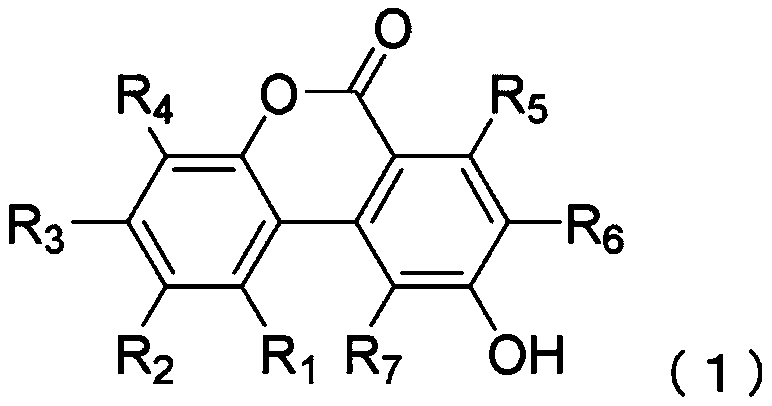
[0230] To ABB medium (manufactured by Oxoid), urolithins (Table 2) having a hydroxyl group at the 9-position of the urolithin skeleton were added as substrates, and then heat-sterilized to replace the gaseous phase with N 2 :CO 2 :H 2 (80% / 10% / 10%) gas, and the medium thus obtained was used as a basic medium. Clostridium baumannii strain JCM 12243 was inoculated in a minimal medium containing each substrate at a final concentration of 1.0 g / L, and cultured anaerobically at 37° C. for 2 weeks. After the culture was completed, 5 mL of the culture solution was extracted with an equal amount of ethyl acetate to extract urolithins, and the obtained ethyl acetate phase was concentrated under reduced pressure and dried to harden. The dried solid obtained above was redissolved in 0.5 mL of methanol, and quantitative ...
Embodiment 2
[0242] Urolithin C, a precursor of urolithin A, was added to ABB medium (manufactured by Oxoid), followed by heat sterilization, and the gas phase was replaced with N 2 :CO 2 :H 2(80% / 10% / 10%) gas, and the culture medium thus obtained was used as a minimal medium. Clostridium baumannii DSM 15670 strain or DSM 29485 strain was inoculated in the minimal medium containing urolithin C at a final concentration of 1.0 g / L, and cultured anaerobically at 37°C. After the culture was completed, 5 mL of the culture solution was extracted with an equal amount of ethyl acetate to extract urolithins, and the obtained ethyl acetate phase was concentrated under reduced pressure and dried to harden. The dried solid obtained above was redissolved in 0.5 mL of methanol, and quantitative analysis of urolithins was performed by HPLC.
[0243] HPLC was carried out under the same conditions as in Example 1. Urolithins manufactured by DALTON PHARMA Co., Ltd. were used as standard products dissolv...
Embodiment 3
[0245] As a result of the same procedure as in Example 2 except that Clostridium asparagus-like DSM 15981 strain was used for 5 days, 95% of the added urolithin C was converted to urolithin A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com