Levetiracetam oral solution and preparation method thereof
An oral solution and solvent technology, applied in the field of medicine, can solve the problems of reducing the bacteriostatic ability of methylparaben and propylparaben, high content of methylparaben and propylparaben, reducing the bacteriostatic effect of preservatives and the like , to achieve the effect of good compliance, low preservative content, and maintaining antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
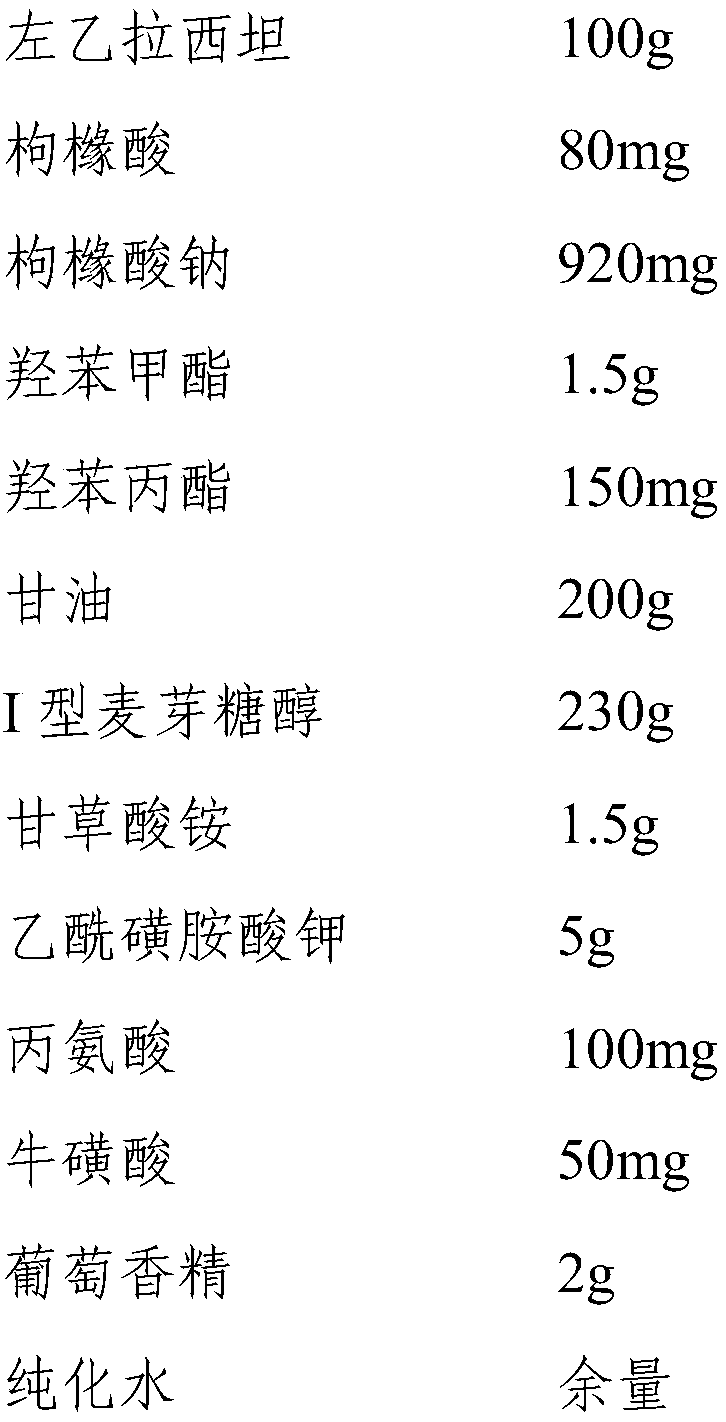
[0043] Embodiment 1 A kind of levetiracetam oral solution
[0044] 1. Prescription composition:
[0045]
[0046] 2. Preparation method:
[0047] Weigh each raw material according to the above ratio for later use, measure 600ml of purified water and heat it to 75°C, add the prescribed amount of glycerin, methylparaben, propylparaben, citric acid, sodium citrate, and type I maltose Alcohol, ammonium glycyrrhizinate, acesulfame potassium, alanine, taurine and levetiracetam, stirred at 2000rpm for 30min, then cooled to 25°C, added the prescribed amount of grape essence, and added purified water to dilute to 1000ml, stirred evenly and then filled to obtain levetiracetam oral solution.
Embodiment 2
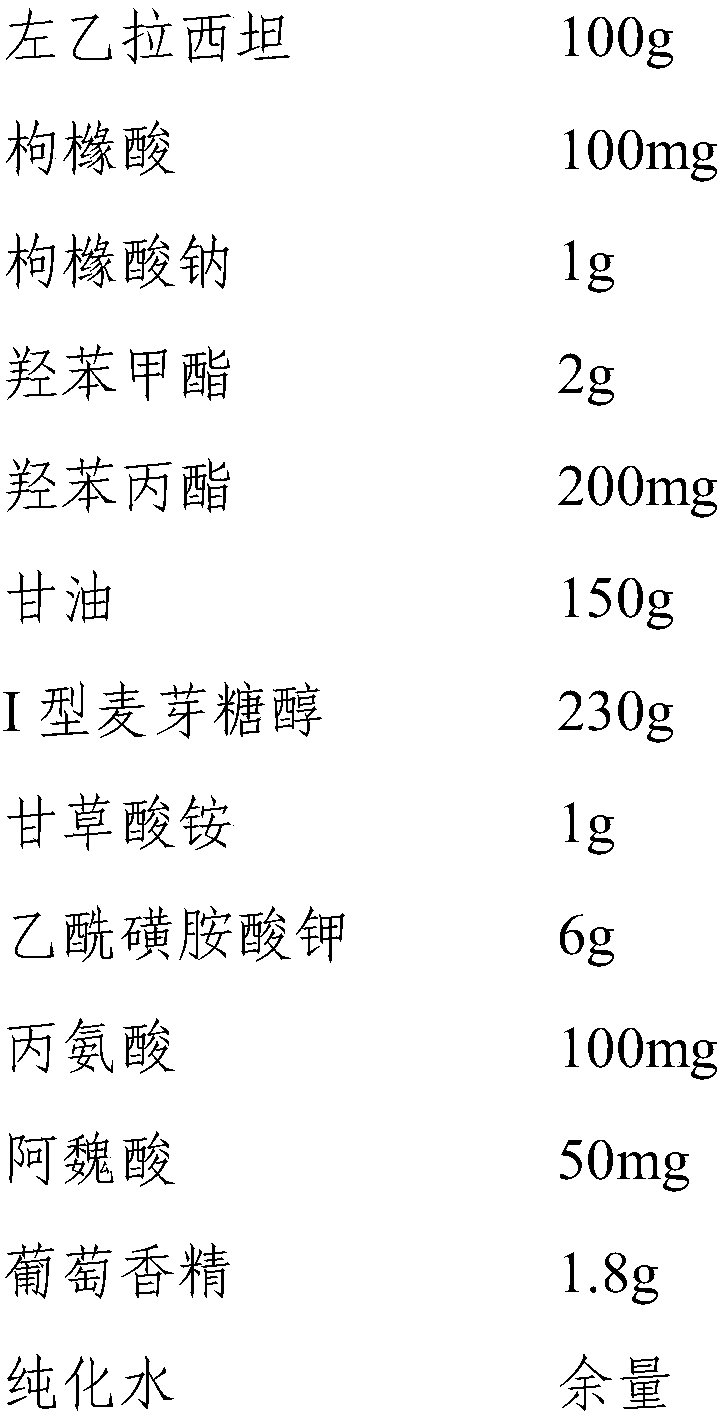
[0048] Embodiment 2 A kind of levetiracetam oral solution
[0049] 1. Prescription composition:
[0050]
[0051] 2. Preparation method:
[0052] Weigh each raw material according to the above ratio for later use, measure 600ml of purified water and heat it to 85°C, add the prescribed amount of glycerin, methylparaben, propylparaben, citric acid, sodium citrate, and type I maltose Alcohol, ammonium glycyrrhizinate, acesulfame potassium, alanine, ferulic acid and levetiracetam, stirred at 1800rpm for 30min, then cooled to 30°C, added the prescribed amount of grape essence, and added purified water to dilute to 1000ml, stirred evenly and then filled to obtain levetiracetam oral solution.
Embodiment 3
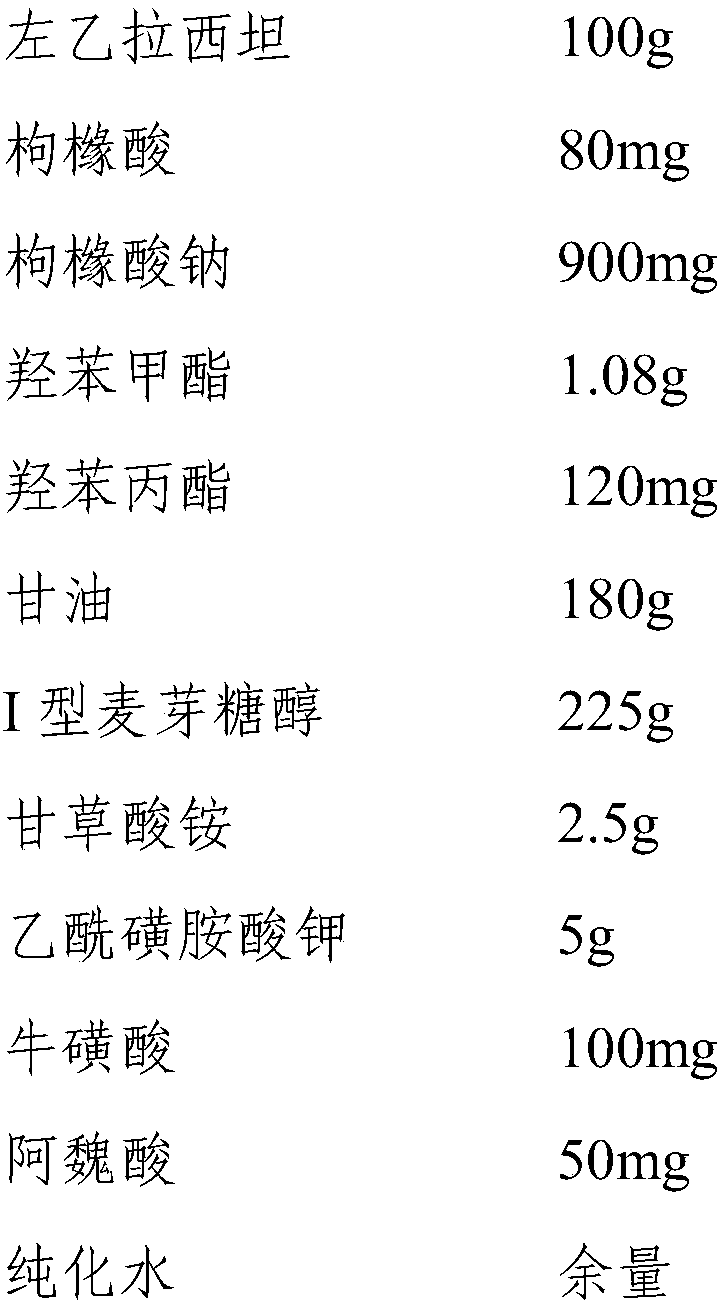
[0053] Embodiment 3 a kind of levetiracetam oral solution
[0054] 1. Prescription composition:
[0055]
[0056] 2. Preparation method:
[0057] Weigh each raw material according to the above ratio for later use, measure 500ml of purified water and heat it to 75°C, add the prescribed amount of glycerin, methylparaben, propylparaben, citric acid, sodium citrate, and type I maltose Alcohol, ammonium glycyrrhizinate, acesulfame potassium, taurine, ferulic acid and levetiracetam, stirred at 1600rpm for 20min, then cooled to 25°C and added purified water to make up to 1000ml, stirred evenly before filling , namely levetiracetam oral solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com