Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
A technology of African swine fever virus and FQ-PCR applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 is used to identify the design of primers and probes for ASFV, CSFV dual TaqMan MGB FQ-PCR detection and the establishment of detection methods
[0050] 1.1 Materials
[0051] 1.1.1 Positive standards and strains
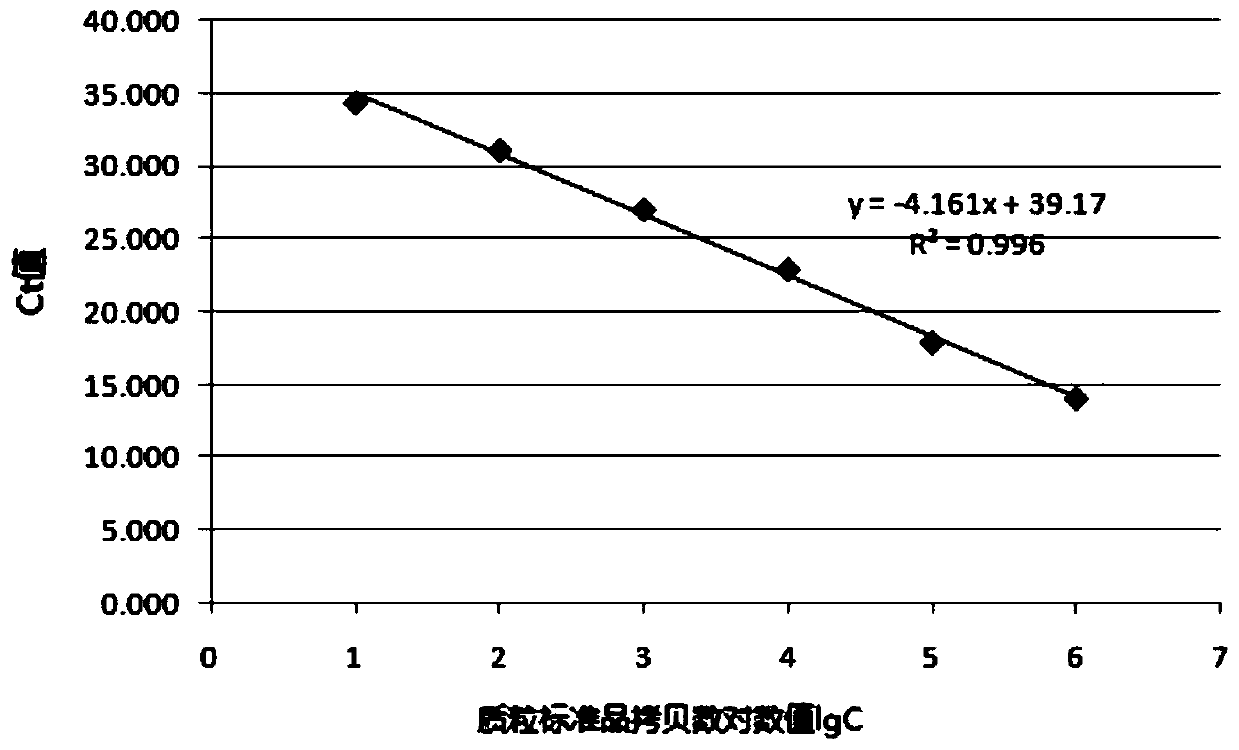
[0052] ASFV and CSFV positive standard samples were amplified by PCR and RT-PCR by the Henan Animal Disease Prevention and Control Center respectively, and the target genes were cloned into the pGEM-T Easy vector to obtain ASFV and CSFV positive standard samples; other controls Viruses, bacteria or positive recombinant plasmids were provided by Henan Animal Disease Prevention and Control Center.
[0053] 1.1.2 Instruments and reagents
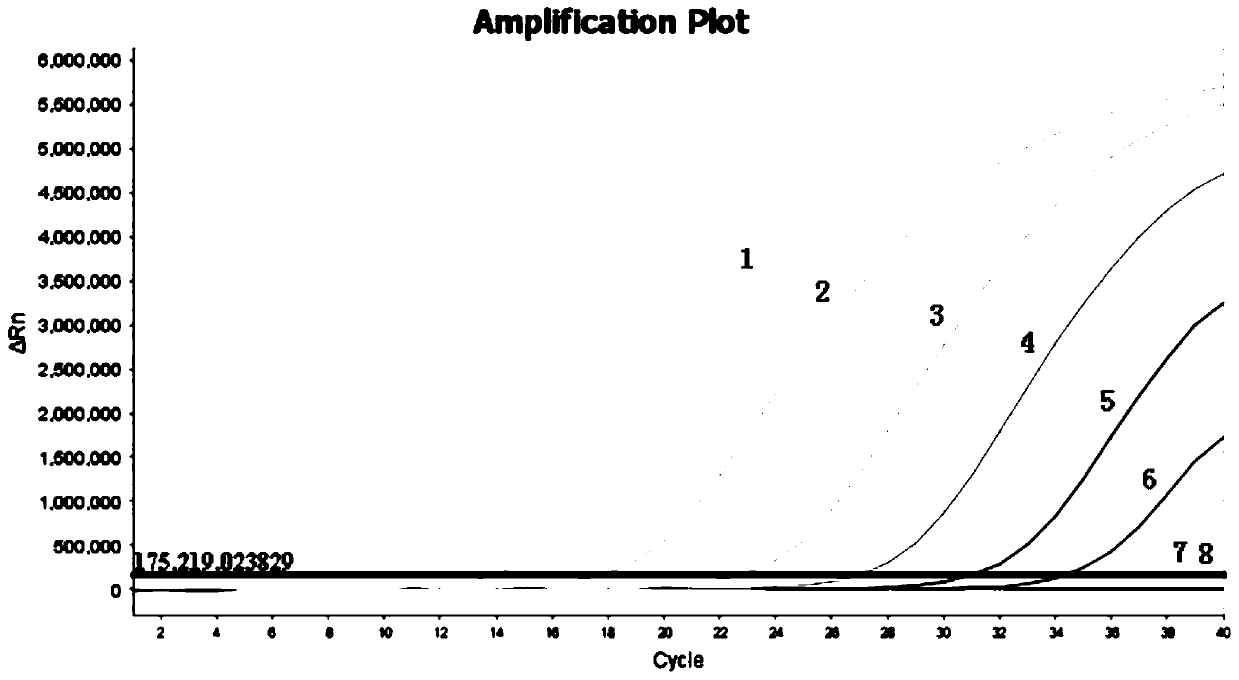
[0054] Fully automatic nucleic acid extraction instrument, product of Thermo Company of the United States; fluorescent PCR instrument, product of ABI Company of the United States; PCR amplification instrument, product of Biometra Company of Germany; gel imaging analysis system, product of Alpha Innotech Company of ...
Embodiment 2
[0100] Example 2 Differentiate ASFV, CSFV double TaqMan MGB FQ-PCR detection kit
[0101] The double TaqMan MGB FQ-PCR detection kit of the present invention includes ASFV detection primer pair and probe (SEQ ID NO: 1-3), CSFV detection primer pair and probe (SEQ ID NO: 4-6).
[0102] The detection kit also includes one or more of PrimeScript RT Master Mix, dNTPs, reverse transcriptase, Taq DNA polymerase, PCR reaction buffer, positive standard, negative control and the like.
[0103] The specific application of the detection kit is as follows:
[0104] (1) Sample requirements
[0105] 1. Apply proper technique to collect samples.
[0106] 2. The sediment and suspended matter in the sample may affect the test results and should be removed by centrifugation.
[0107] 3. Samples should not be placed at room temperature for more than 6 hours after processing and collection; if not tested within 6 hours, the samples should be placed in a refrigerator at 2-8°C; if they need to b...
Embodiment 3
[0131] Example 3 Distinguish the application of ASFV, CSFV dual TaqMan MGB FQ-PCR detection technology
[0132] The dual TaqMan MGB FQ-PCR detection method for distinguishing ASFV and CSFV established according to the present invention is compared with the conventional PCR detection method for ASFV and CSFV established before, with ASFV and CSFV positive standards as positive controls; sterilized PBS solution as negative In contrast, 10 clinical suspected ASFV or CSFV infection samples (sample numbers are: 1~10) adopt the method provided by the present invention and common PCR / RT-PCR (reverse transcription PCR) detection to carry out laboratory detection respectively, and these The detection results of the two methods were compared and analyzed, and compared with the sequencing results. The results are shown in Table 3. Result shows, adopt the double TaqMan MGB FQ-PCR method detection of distinguishing ASFV provided by the present invention, CSFV, 3 parts are ASFV positive, 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com