Anti-adrenomedullin (ADM) antibody or Anti-adm antibody fragment or Anti-adm non-ig scaffold for use in intervention and therapy of congestion in a patient in need thereof
An adrenomedullin, antibody fragment technology, applied in the direction of antibodies, antibody medical components, blood diseases, etc., can solve harmful problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
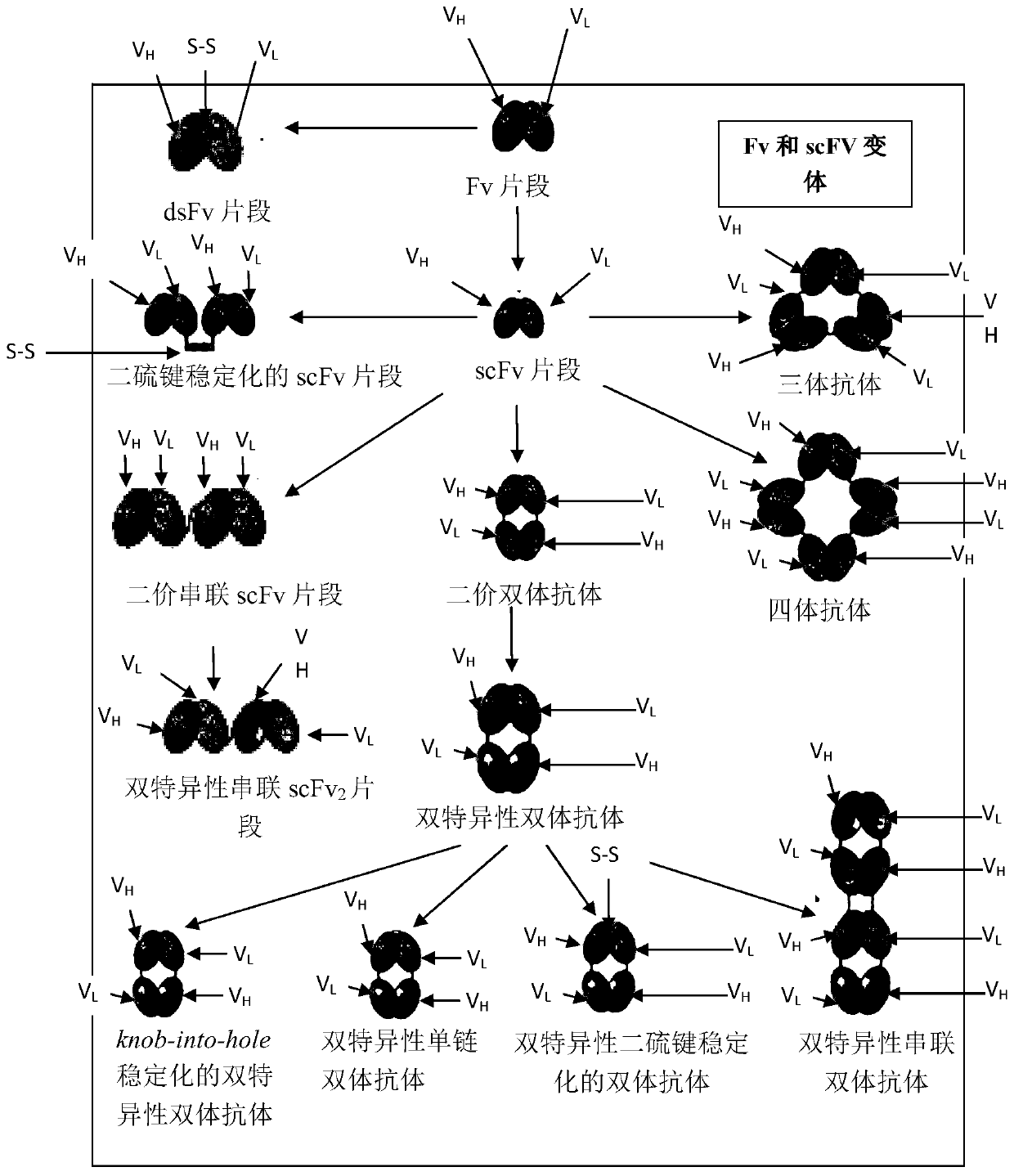
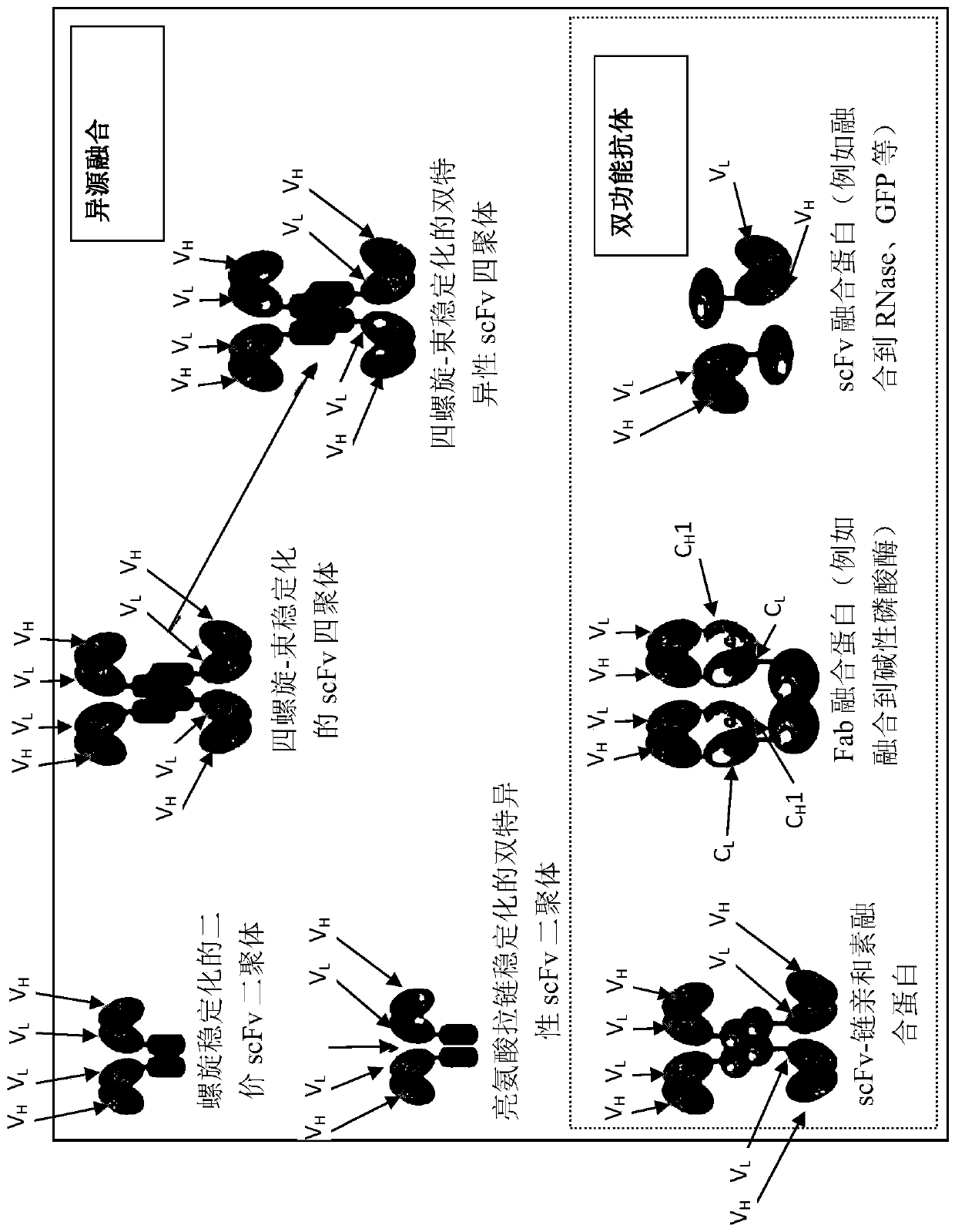
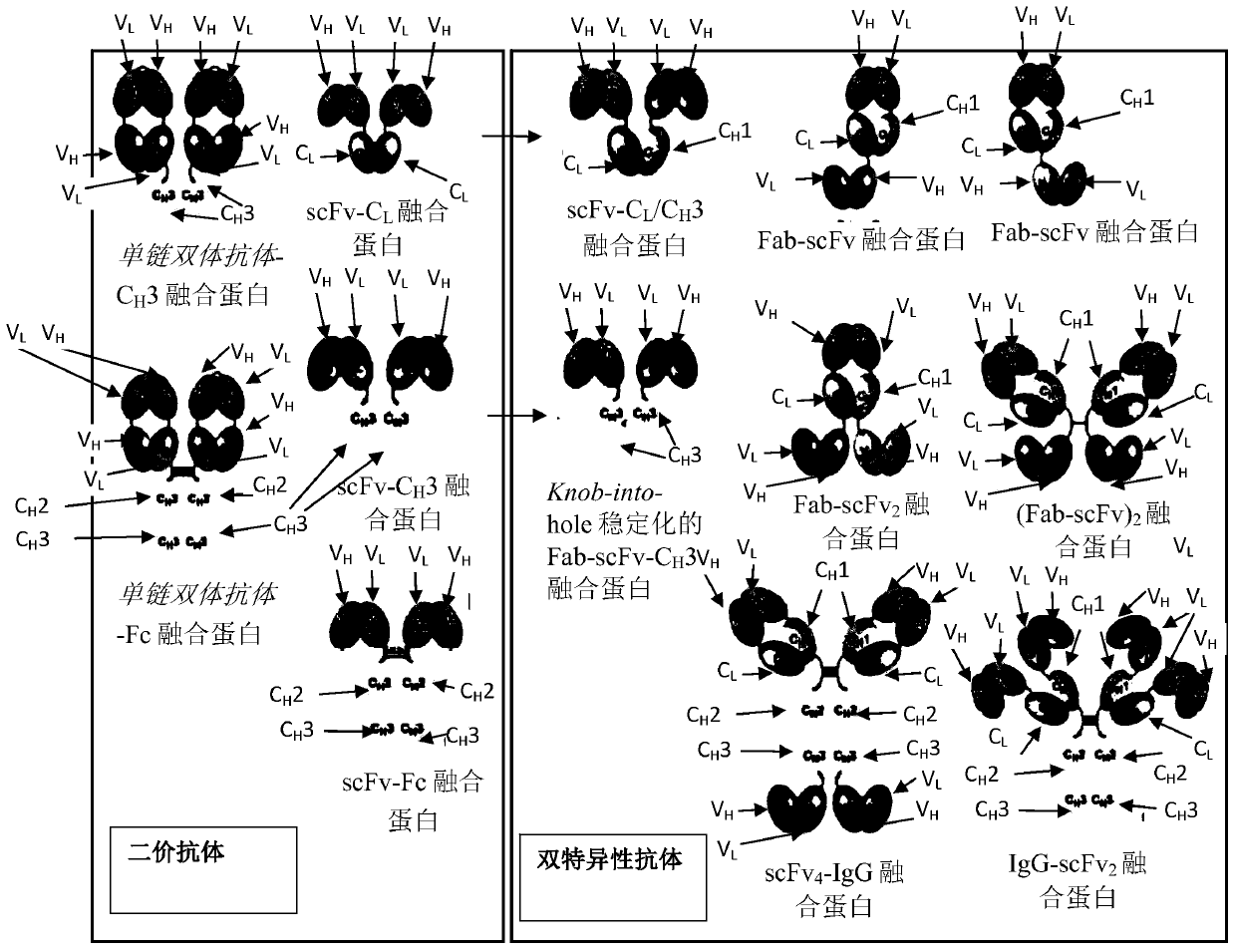
Image
Examples
Embodiment 1
[0347] Production of antibodies and determination of their affinity constants
[0348] Several human and murine antibodies were generated and their affinity constants determined (see Tables 1 and 2).
[0349] Peptides / Conjugates for Immunization:
[0350] Peptides for immunization were synthesized, see Table 1 (JPT Technologies, Berlin, Germany) with an additional N-terminal cysteine (if no cysteine is present within the chosen ADM sequence) residue Used to couple the peptide to bovine serum albumin (BSA). The peptides were covalently linked to BSA using Sulfolink coupling gels (Perbio-science, Bonn, Germany). The coupling procedure was performed according to the manual of Perbio.
[0351]Mouse antibodies were generated as follows:
[0352] Balb / c mice were treated with 100 μg of peptide-BSA conjugate (emulsified in 100 μl Freund’s complete adjuvant) on days 0 and 14 and 50 μg (in 100 μl Freund’s incomplete adjuvant) on days 21 and 28 Immunization. Three days prior t...
Embodiment 2
[0403] Effect of selected anti-ADM antibodies on anti-ADM biological activity
[0404] The effect of selected ADM antibodies on ADM biological activity was tested in a human recombinant adrenomedullin receptor cAMP functional assay (adrenomedullin bioassay).
[0405] Testing of Antibodies Targeting Human or Mouse Adrenomedullin in Human Recombinant Adrenomedullin Receptor cAMP Functional Assay (Adrenomedullin Bioassay)
[0406] Material:
[0407] Cell line: CHO-K1
[0408] Receptor: Adrenomedullin (CRLR+RAMP3)
[0409] Recipient cell line accession numbers: CRLR: U17473; RAMP3: AJ001016
[0410] CHO-K1 cells expressing human recombinant adrenomedullin receptor (FAST-027C) grown in antibiotic-free medium prior to the assay were detached by gentle washing with PBS-EDTA (5 mM EDTA) and recovered by centrifugation , and resuspended in assay buffer (KRH: 5mM KCl, 1.25mM MgSO 4 , 124mM NaCl, 25mM HEPES, 13.3mM Glucose, 1.25mM KH 2 PO 4 , 1.45mM CaCl 2 , 0.5g / l BSA).
[04...
Embodiment 3
[0420] Stabilization of hADM data by anti-ADM antibody
[0421] The stabilizing effect of human ADM antibodies on human ADM was tested using the hADM immunoassay.
[0422] Immunoassay for the quantification of human adrenomedullin
[0423] The technique used is based on acridinium ester labeled sandwich-coated tube luminescence immunoassay.
[0424] Labeled compounds (tracers):
[0425] 100 μg (100 μl) of CT-H (1 mg / ml in PBS, pH 7.4, AdrenoMed AG Germany) was mixed with 10 μl of acridine NHS ester (1 mg / ml in acetonitrile, InVent GmbH, Germany) (EP 0353971), And incubate at room temperature for 20min. Marked CT-H in Purification was performed by gel filtration HPLC on SEC 400-5 (Bio-Rad Laboratories, Inc., USA). The purified CT-H was diluted in (300mmol / L potassium phosphate, 100mmol / L NaCl, 10mmol / L Na-EDTA, 5g / L bovine serum albumin, pH 7.0). The final concentration of labeled compound was about 800.000 relative light units (RLU) per 200 μL (about 20 ng of labeled an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com