A kind of two-photon ultra-low background fluorescent probe and its preparation method and application
A fluorescent probe and two-photon technology, applied in the field of analytical chemistry, can solve the problems of large background fluorescence interference, complex processing process, and large cell damage, and achieve the effects of improving detection sensitivity, reducing interference, and reducing sample damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
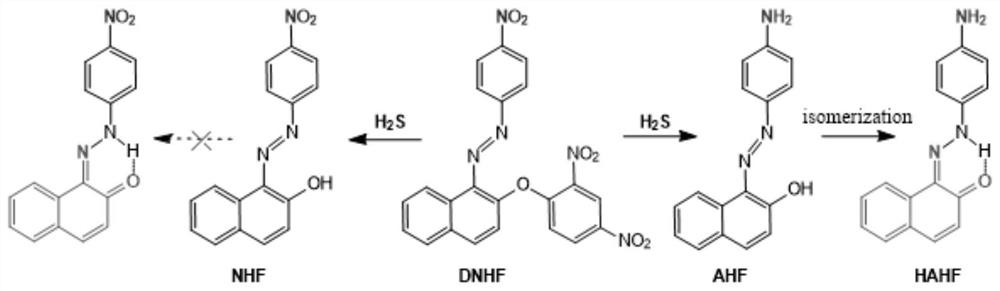
[0068] Preparation of NDN
[0069] (1) Preparation of diazonium salt
[0070] 1) Add 5ml distilled water and 0.5ml concentrated sulfuric acid in the round bottom flask;
[0071] 2) Then add 0.5g p-nitroaniline and stir under ice bath;
[0072] 3) Finally, 0.25 g of sodium nitrate was added to 1 ml of distilled water, slowly added to the solution prepared in step 2), and the synthesis of NDN was continued for 5 h under ice-bath stirring.
[0073] 1) 2-naphthol 1g (7mmol) solution, add 5ml of 1M sodium hydroxide. The solvent used needs to be deoxygenated by argon gas bubbling, and then further treated with molecular sieves to obtain an anhydrous and oxygen-free solvent.
[0074] 2) Add 1 g (6.7 mmol) of diazonium salt in an ice bath and stir.
[0075] 3) The mixture was acidified with 0.5M sulfuric acid, and the product was vacuum-dried at 40-60° C. for 2 hours to obtain the product NDN.
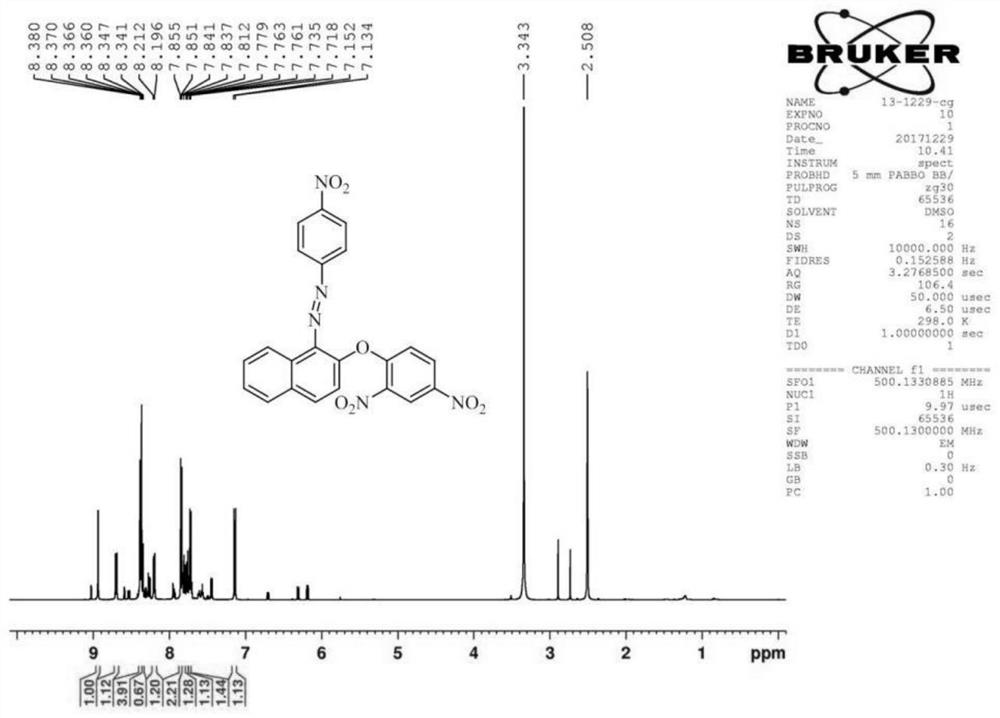
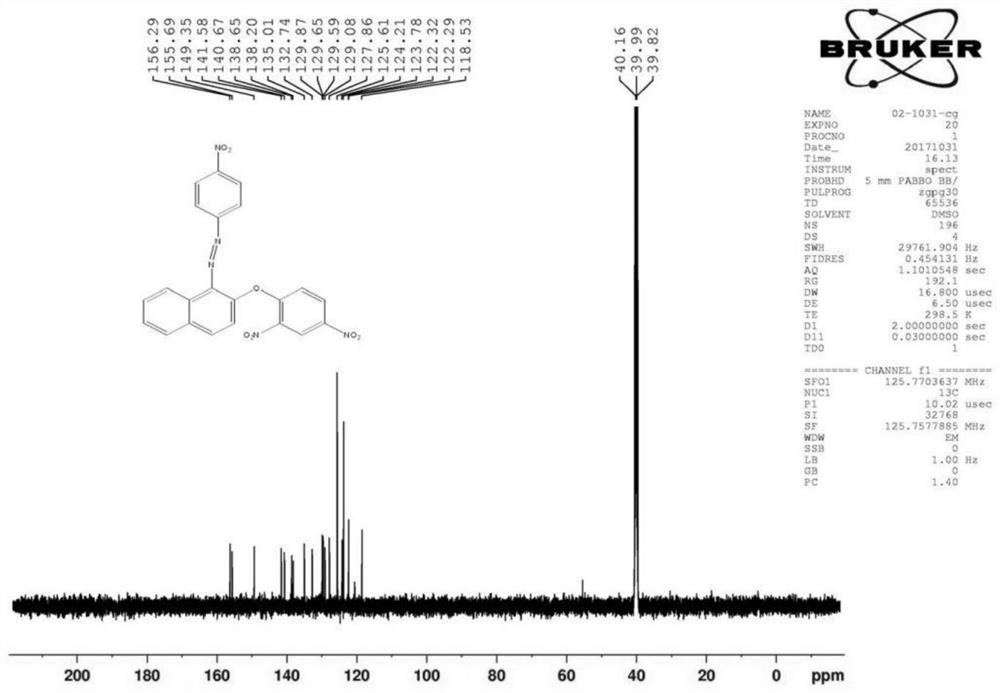
[0076] (2) Fluorescent probe DNHF-H 2 Preparation of S
[0077] 1) The experiment w...
Embodiment 2
[0082] Preparation of NDN
[0083] (1) Preparation of diazonium salt
[0084] 3) Add 10ml distilled water and 1.0ml concentrated sulfuric acid in the round bottom flask;
[0085] 4) Then add 1.0g p-nitroaniline and stir under ice bath;
[0086] 3) Finally, add 0.5g of sodium nitrate to 2ml of distilled water, slowly add to the solution prepared in step 2), and continue to stir for 5h under ice bath
[0087] Synthesis of NDNs.
[0088] 1) 2-naphthol 1.5g (10mmol) solution, add 3M sodium hydroxide 7.5ml. The solvent used needs to be deoxygenated by argon gas bubbling, and then further treated with molecular sieves to obtain an anhydrous and oxygen-free solvent.
[0089] 2) Add 1.5 g (10 mmol) of diazonium salt in an ice bath, and stir.
[0090] 3) The mixture was acidified with 1M sulfuric acid, and the product was vacuum-dried at 40-60° C. for 2 hours to obtain the product NDN.
[0091] (2) Fluorescent probe DNHF-H 2 Preparation of S
[0092] 1) The experiment was carri...
Embodiment 3
[0097] Preparation of NDN
[0098] (1) Preparation of diazonium salt
[0099] 1) Add 10ml of distilled water and 1.5ml of concentrated sulfuric acid in a round bottom flask;
[0100] 2) Then add 1.5g p-nitroaniline and stir under ice bath;
[0101] 3) Finally, add 1g of sodium nitrate to 3ml of distilled water, slowly add to the solution prepared in step 2), and continue to stir for 5h under ice bath
[0102] Synthesis of NDNs.
[0103] 1) 2-naphthol 2g (14mmol) solution, add 10ml of 5M sodium hydroxide. The solvent used needs to be deoxygenated by argon gas bubbling, and then further treated with molecular sieves to obtain an anhydrous and oxygen-free solvent.
[0104] 2) Add 2 g (13.3 mmol) of diazonium salt in an ice bath, and stir.
[0105] 3) The mixture was acidified with 1.5M sulfuric acid, and the product was vacuum-dried at 40-60° C. for 2 hours to obtain the product NDN.
[0106] (2) Fluorescent probe DNHF-H 2 Preparation of S
[0107] 1) The experiment was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com