Nucleic acid and application thereof
A nucleic acid and nucleotide technology, applied in the field of biomedicine, can solve problems such as low potency, short action time, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
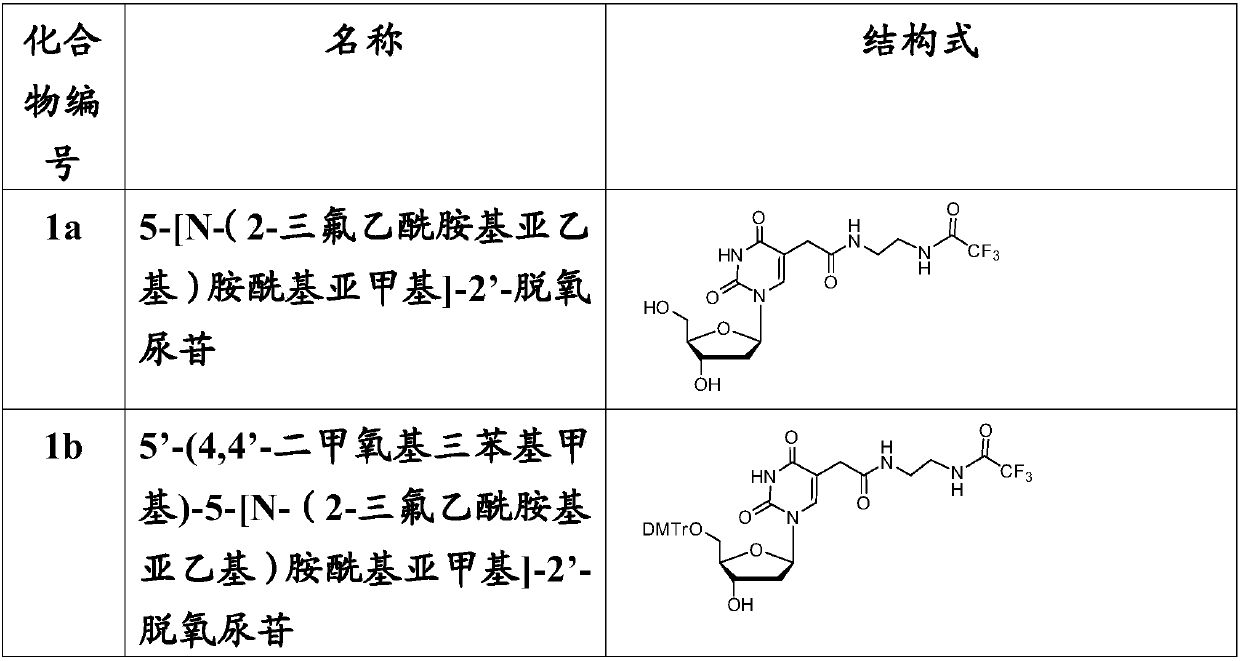
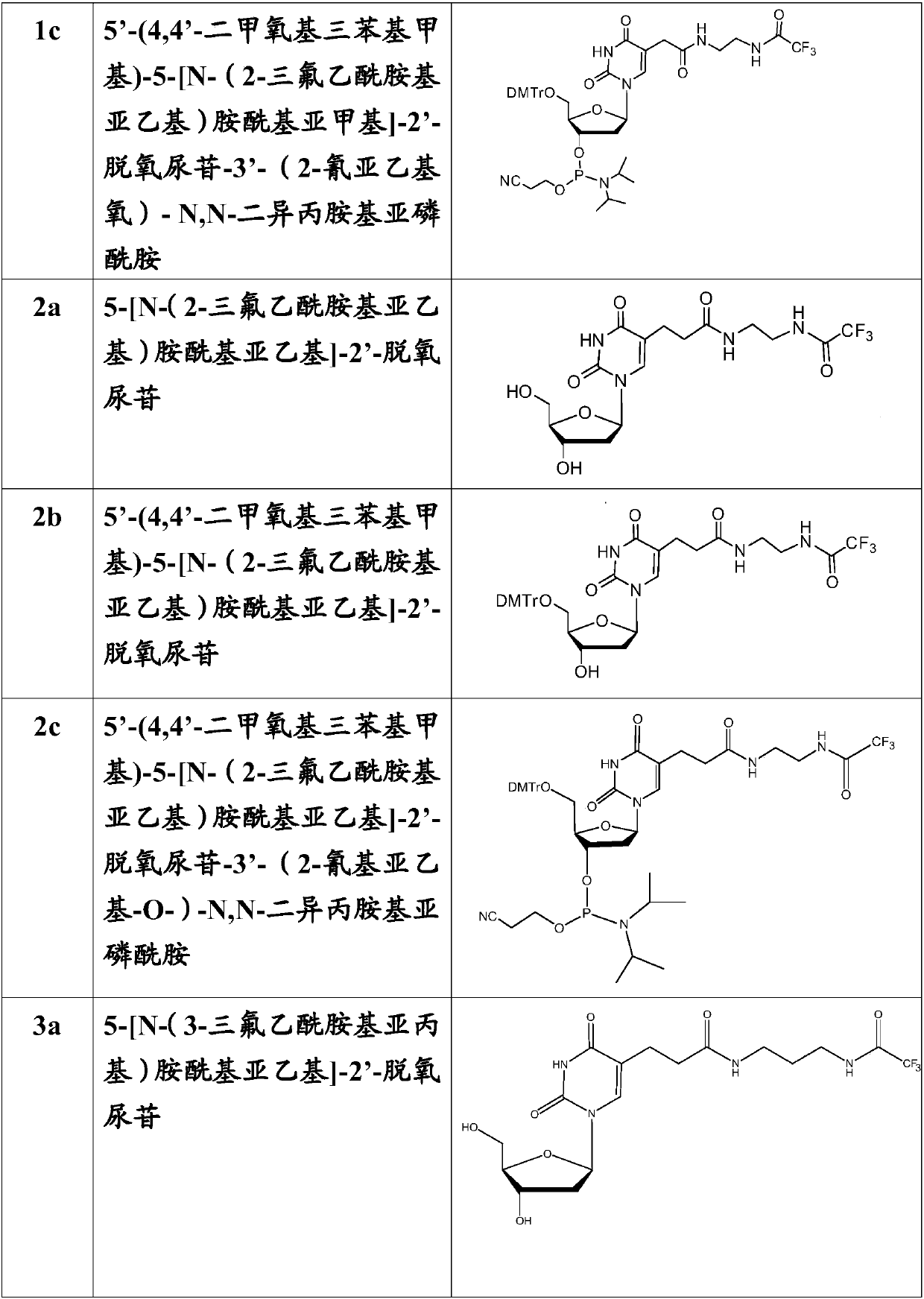
[0100] Example 1: 5-[N-(2-trifluoroacetamidoethylene)aminoacylmethylene]-2'-deoxyuridine (compound Synthesis of 1a)
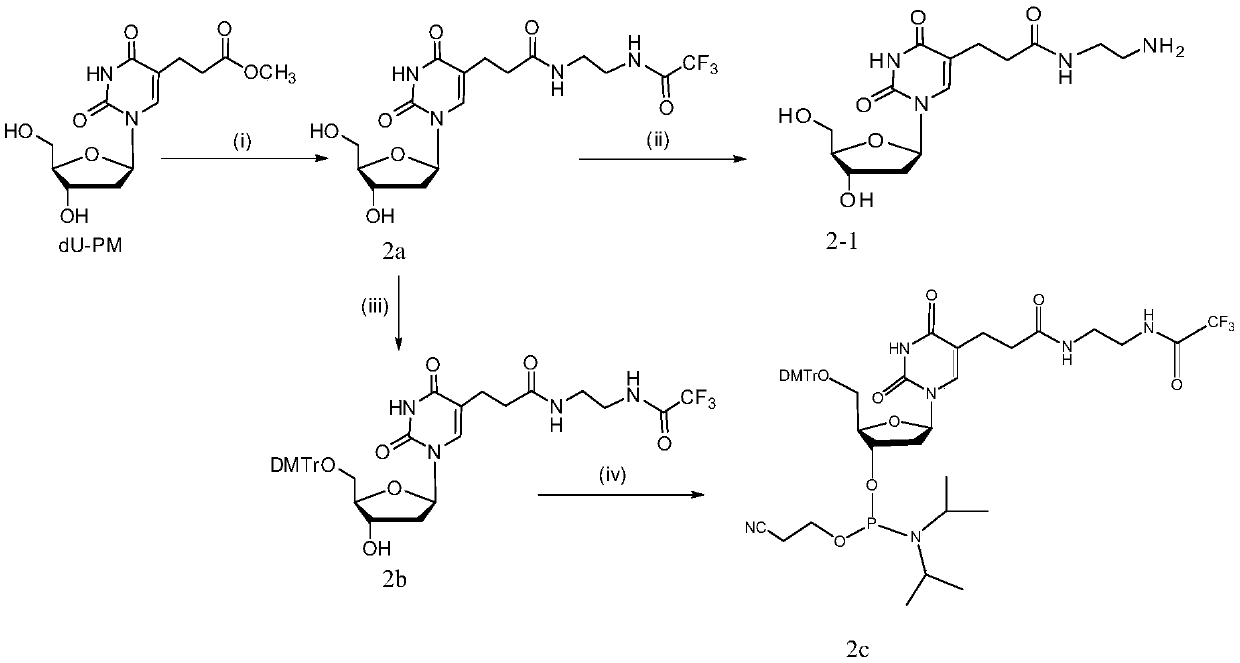
[0101]According to the reaction formula in Scheme 1, 1.14g of 5-methoxycarbonylmethylene-2'-deoxyuridine (dU-EM, 3.8mol) was dissolved in 5mL of methanol, and it was added to 2.5mL of ethylenediamine (38mmol ) methanol solution, stirred for 4 hours, thin layer chromatography (TLC) showed that the reaction was complete. The reaction mixture was dissolved in 10mL of methanol, then 2.1mL of triethylamine and 5mL of ethyl trifluoroacetate were added to mix, the mixture was thoroughly mixed with silica gel and the solvent was evaporated under reduced pressure, and the product 1.1g (compound 1a), yield 68.3%, R f (dichloromethane:methanol, 9:1) 0.32.
[0102] 1 H NMR (400MHz, DMSO-d 6 ):δ2.08(m,2H,C2'-H),3.05(s,2H,CH 2 ),3.18(m,4H,CH 2 CH 2 ),3.56(m,2H,C5'-H),3.77(m,1H,C4'-H), 4.22(m,1H,C3'-H),4.96(t,J=5.5,C5'-OH ), 5.24(d, J=4.2, C3'-OH), 6.17(t, J=4.8, C...
Embodiment 2
[0105] Example 2: Synthesis of 5-(2-aminoethyl)-aminoacyl methylene-2'-deoxyuridine (compound 1-1)
[0106] According to the reaction formula in scheme 1, compound 1a (400mg, 0.94mmol) was added into concentrated ammonia water (40mL), stirred at room temperature for 4 hours, TLC showed that the reaction was complete. Column chromatography obtains product 286mg, yield 93%, R f (dichloromethane / ammonia methanol=1:1) 0.47.
[0107] 1 H NMR (400MHz, DMSO-d 6 ):2.09(m,2H,C2'-H),2.85(m,2H,CH 2 ),3.10(s,2H,CH 2 ),3.27(m,2H,CH 2 ),3.53(m,2H,C5'-H),3.78 (m,1H,C4'-H),4.24(m,1H,C3'-H),5.31(br,1H,C3'-OH), 6.18(m,1H,C1'-H),7.77(s,1H,C6-H),8.09(m,1H,NH),8.55(br,1H,NH).
[0108] 13 C NMR (100MHz, DMSO-d 6 ):33.9,37.1,61.9,70.9,84.5,87.9,109.0,139.0,150.9,163.8,170.8.
[0109] HRMS (C 13 h 20 N 4 o 6 +H + ,329.1456):329.1456; (C 13 h 20 N 4 o 6 +Na + , 351.1275):351.1276.
Embodiment 3
[0110] Example 3: 5'-(4,4'-dimethoxytriphenylmethyl)-5-[N-(2-trifluoroacetamidoethylene)amide Synthesis of Methylene]-2'-deoxyuridine (compound 1b)
[0111] According to the reaction formula in route 1, 1.20g of compound 1a (2.82mmol) was dissolved in 5mL of dry pyridine, 1.16g of DMTr-Cl (3.38mmol) was added thereto, and reacted at room temperature for 2 hours, when TLC showed that the reaction was complete , separated by column chromatography (5% pyridine treated silica gel) to obtain product 1.28g (compound 1b), yield 62.5%, R f (dichloromethane:methanol, 9:1) 0.56.
[0112] 1 H NMR (400MHz, DMSO-d 6 ):δ2.18(m,2H,C2'-H),2.70(s,2H,CH 2 ),3.17(m,6H,CH 2 CH 2 ,C5'-H),3.73(s,6H,2OCH 3 ), 3.87(m, 1H, C4'-H), 411.25(m, 1H, C3'-H), 5.32(d, J=4.5, C3'-OH), 6.20(t, J=4.8, C1' -H),6.86,7.19-7.40(2m,13H,arom.H),7.55(s,1H,C6-H),7.86(m,1H,CONH),9.33(s,1H,CONHCOCF 3 ),11.38(s,1H,3-NH).
[0113] 13 C NMR (400MHz, DMSO-d 6 ):δ33.1,37.5,55.0,63.9,70.5,84.0,85.4,85.8,108.8,113...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com