Preparation method of compound containing 6-methyl uracil structure
A compound, dimethylformamide technology, applied in the field of preparation of compounds containing 6-methyluracil structure, can solve the problems of unfavorable industrial scale-up production, high price, low overall yield, etc., and achieve easy scale-up production and operation Simple, high reaction yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
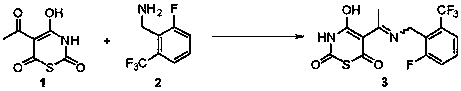
[0027] Compound 1 (5.33 g), isopropanol (50 mL) and compound 2 (5 g) were added into a 100 mL three-neck flask, and the reaction was heated at 80-90 °C. After the reaction was completed, water (100 mL) was added and extracted with dichloromethane. The organic phases were combined, the solvent was removed by rotary evaporation, and 9.2 g of compound 3 was obtained by column purification, with a yield of 98% and a purity of 99.5%.
[0028] The NMR data of compound 3 are as follows:
[0029] 1 H NMR (400 MHz, DMSO) δ 12.91 (s, 1H), 11.66 (s, 1H), 7.73 (dd, J = 7.4,4.5 Hz, 3H), 4.94 (d, J = 5.0 Hz, 2H), 2.69 (s, 3H).
Embodiment 2
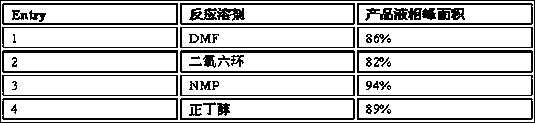
[0031] Add compound 1 and compound 2 into different reaction vials, and then add the following solvents respectively, heat the oil bath to 80-90 °C for 3 h, and take a sample for liquid chromatography. The liquid phase peak area test results of the product compound 3 are as follows.
[0032]
Embodiment 3
[0034]
[0035] Add compound 3 (5 g) and DMF (25 mL) into a 100 mL three-necked flask. Heating to 120~130 °C to react. After the reaction was completed, water (50 mL) was slowly added to precipitate a solid, which was filtered to obtain compound 4. Yield 82.5%, purity 98.0%.
[0036] The NMR data of compound 4 are as follows:
[0037] 1 H NMR (400 MHz, CDCl 3 ) δ 9.03 (s, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.46-7.41 (m, 1H), 7.30-7.25 (m, 1H), 5.61 (s, 1H), 5.38 (s, 2H), 2.17 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com