Side chain fatty acid modified antimicrobial peptide analogues containing D-type amino acid and synthesis and application thereof
A technology of amino acids and fatty acids, applied in the field of biochemistry, can solve problems such as high manufacturing cost, intolerance of physiological conditions, and limitation of clinical application of AMPs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
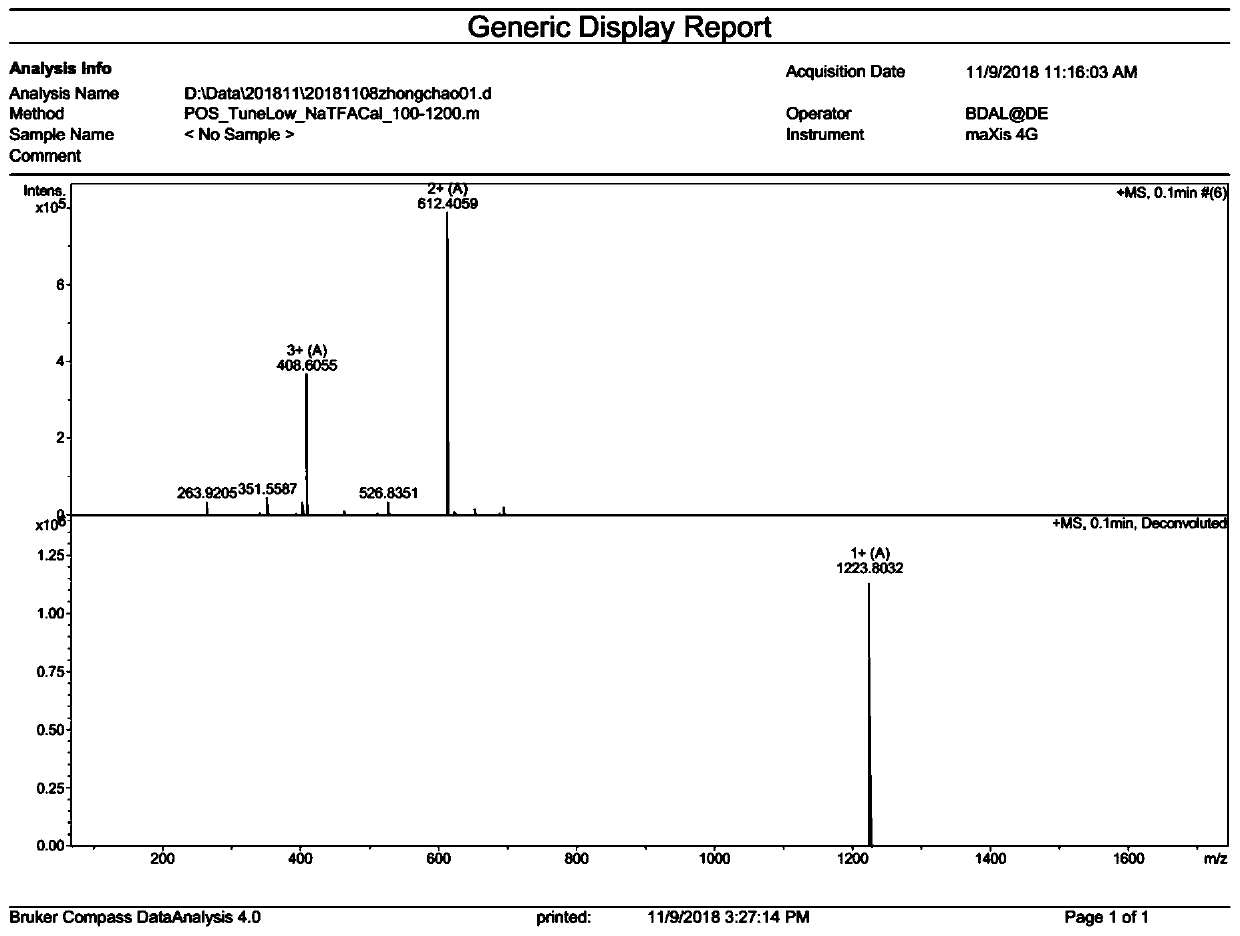
[0054] Example 1: Ano-D4,7-4C 4 Synthesis
[0055] (1) Activation and pretreatment of resin
[0056] Accurately weigh 0.47g of MBHA resin (0.43mmol / g) and place it in a peptide solid-phase synthesizer. After the DCM solution swells for 30 minutes, the resin is colorless and transparent through the ninhydrin color test, indicating that the resin is normal.
[0057] (2) Synthesis of Fmoc-Ano-D4,7-4(Mtt)-resin
[0058] For the above-mentioned normal MBHA resin, the Fmoc protecting group was removed by the DMF solution containing 20% piperidine by volume fraction, and the ninhydrin chromogenic method was used to detect that the resin was blue-purple, indicating that the protecting group had been removed; the Fmoc-Leu-OH (212mg), HOBT (81mg), HBTU (228mg), and DIEA (0.2mL) were dissolved in 5-10mL DMF and mixed evenly, added to the synthesizer and mixed with the above-mentioned MBHA resin from which the Fmoc protecting group was removed, and condensed for 1 hour; Triketone chr...
Embodiment 2
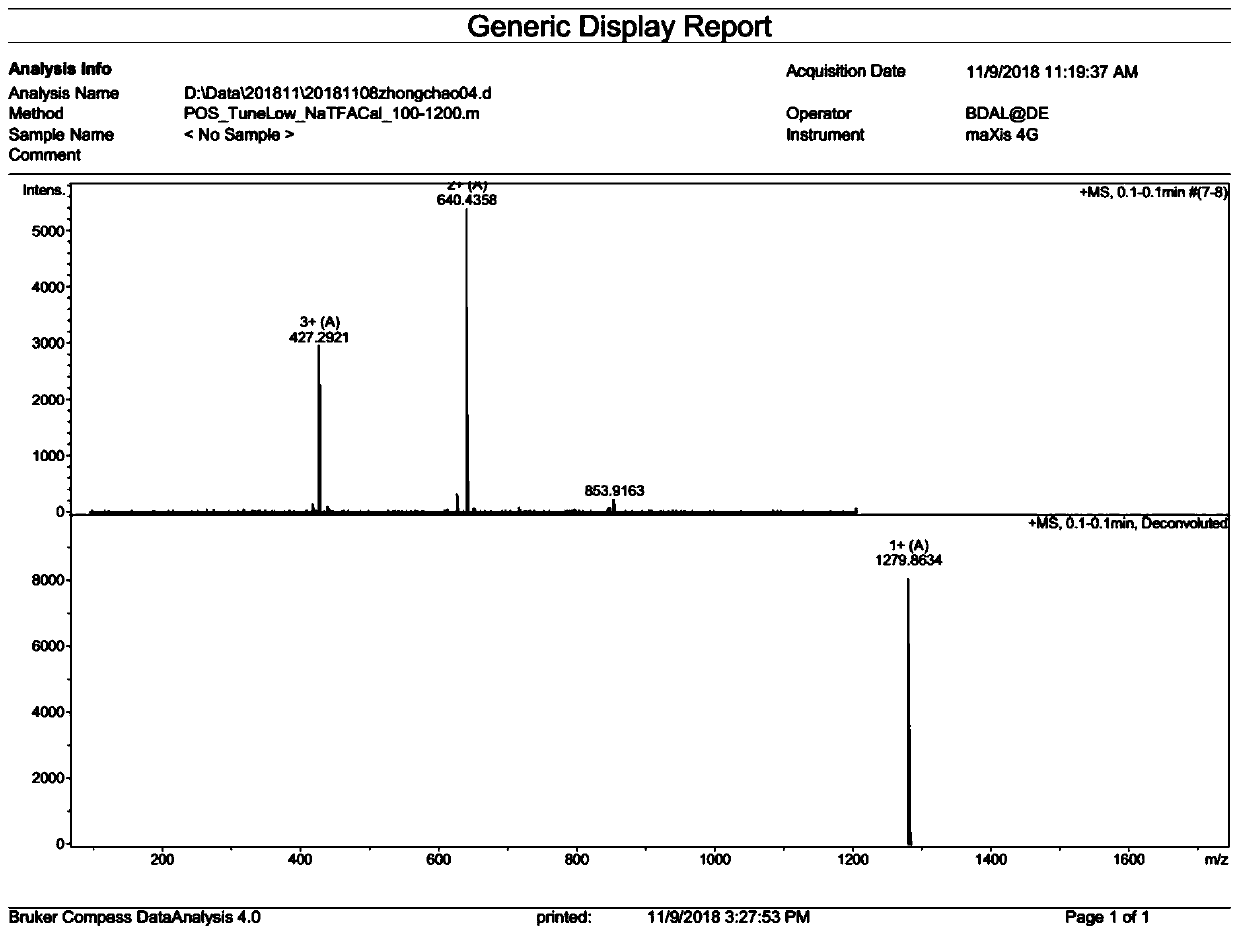
[0065] Example 2: Ano-D4,7-4C 8 Synthesis
[0066] (1) Activation and pretreatment of resin
[0067] With embodiment 1.
[0068] (2) Synthesis of Fmoc-Ano-D4,7-4(Mtt)-resin
[0069] With embodiment 1.
[0070] (3)Ano-D4,7-4C 8 Synthesis of -resin
[0071] The above-obtained Fmoc-Ano-D4,7-4(Mtt)-resin was removed with a DCM solution containing 1% TFA by volume to remove the side chain Mtt protecting group, and the ninhydrin colorimetric method showed that the resin was blue-purple. Indicates that the protecting group has been removed to obtain Ano-D4,7-4(NH 2 )-resin; Octanoic anhydride (C n , n=8; 1.53mL), HOBT (81mg), HBTU (228mg), DIEA (0.2mL) were dissolved and mixed in 5-10mL DMF, and added to the synthesizer with the above-mentioned Ano- D4,7-4(NH 2 )-resin mixed, condensation reaction 1.5h; ninhydrin color test, the resin was colorless and transparent, indicating that the condensation reaction was complete, and Ano-D4,7-4C was obtained 8 -resin; with the DMF so...
Embodiment 3
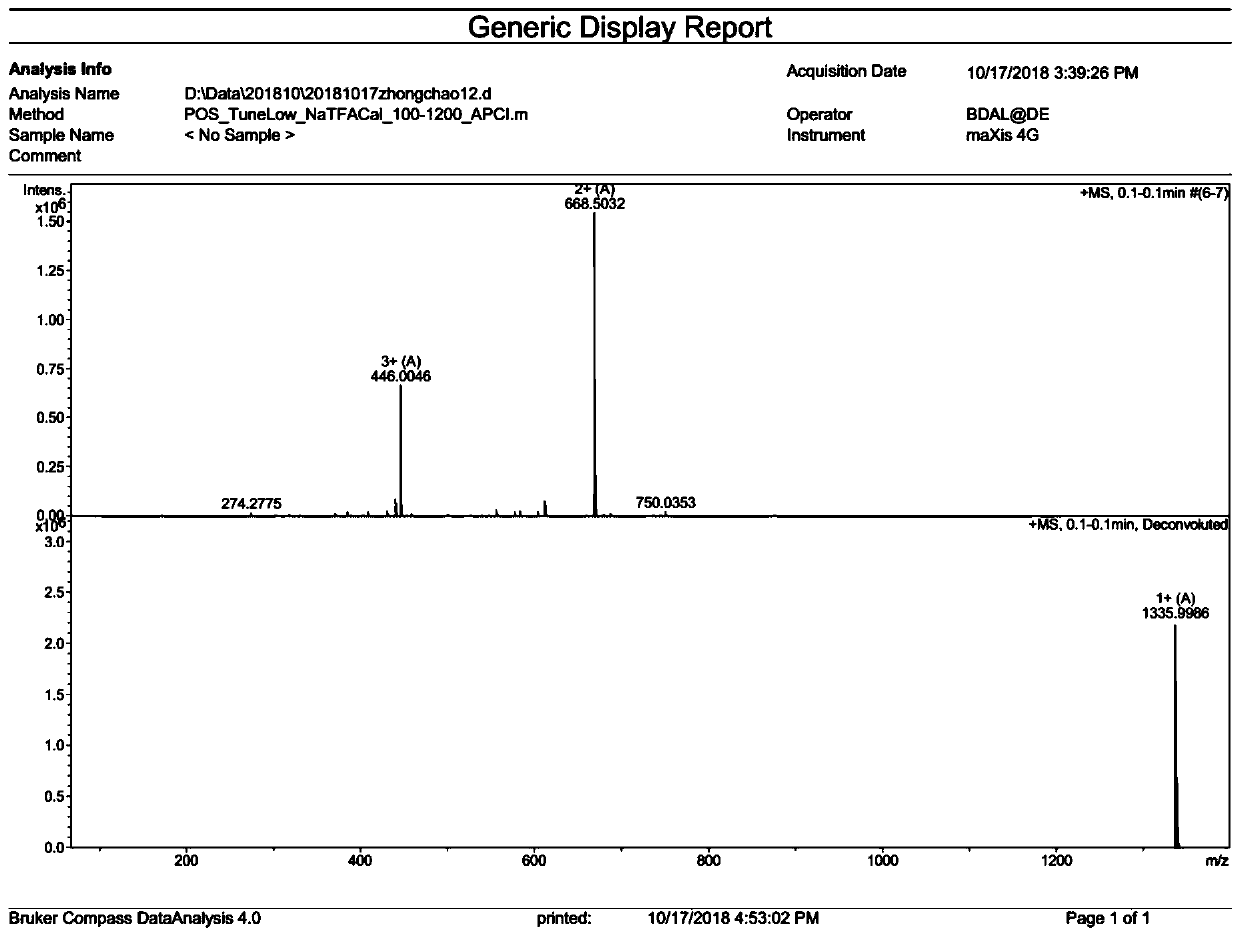
[0076] Example 3: Ano-D4,7-4C 12 Synthesis
[0077] (1) Activation and pretreatment of resin
[0078] With embodiment 1.
[0079] (2) Synthesis of Fmoc-Ano-D4,7-resin
[0080] With embodiment 1.
[0081] (3)Ano-D4,7-4C 12 Synthesis of -resin
[0082] The above-obtained Fmoc-Ano-D4,7-4(Mtt)-resin was removed with a DCM solution containing 1% TFA by volume to remove the side chain Mtt protecting group, and the ninhydrin colorimetric method showed that the resin was blue-purple. Indicates that the protecting group has been removed to obtain Ano-D4,7-4(NH 2 )-resin; Dodecanoic acid (C n , n=12; 240mg), HOBT (81mg), HBTU (228mg), DIEA (0.2mL) were dissolved and mixed in 5-10mL DMF, and added to the synthesizer with the above-mentioned Ano-D4 with the side chain Mtt protection group removed ,7-4(NH 2 )-resin mixed, condensation reaction 1.5h; ninhydrin color test, the resin was colorless and transparent, indicating that the condensation reaction was complete, and Ano-D4,7-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com