N-phenyl-N-p-toluenesulfonyl trifluoroacetamide (NTFTS) and application
A technology of tosyl trifluoroacetamide and trifluoroacetophenone is applied in the field of pharmaceutical chemical intermediates and related chemistry, can solve the problems of low atom economy, chemical waste and the like, achieves environmental friendliness, low reaction cost, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
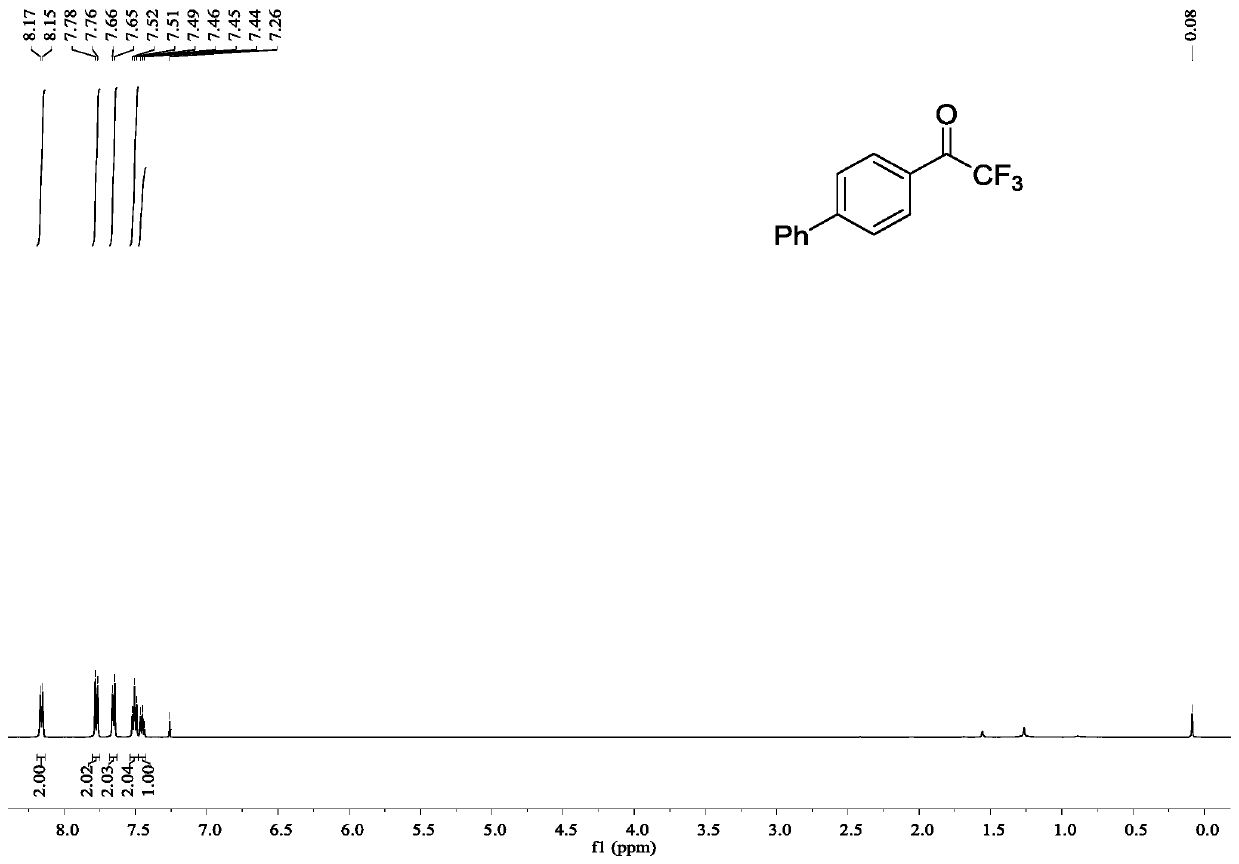
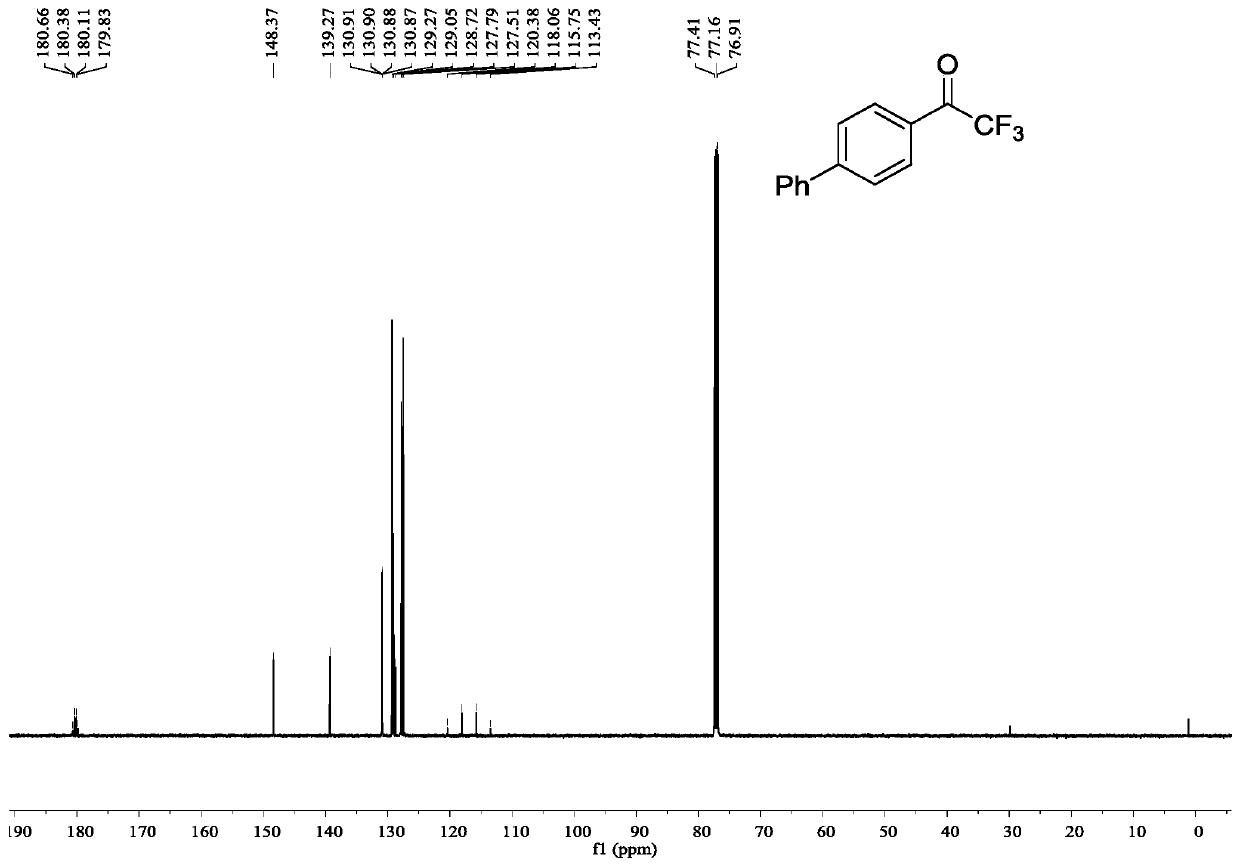
[0040] Example 1: Synthesis of 2,2,2-trifluoro-1-((1,1'-biphenyl-4-yl)ethanone
[0041] In a 25mL reactor, add palladium acetate (0.0023g, 0.01mmol), potassium carbonate (0.0553g, 0.4mmol), tri-tert-butylphosphine (0.0041g, 0.02mmol) and NTFTS (0.0687g, 0.2mmol), nitrogen After three replacements, 2 mL of anhydrous toluene was added, 4-biphenylboronic acid (0.0792 g, 0.4 mmol) was added with stirring, and stirred at 25°C for 24 h. Column chromatography (silica gel, 200-300 mesh; developer, petroleum ether: ethyl acetate = 100:1) to obtain 2,2,2-trifluoro-1-((1,1'-biphenyl-4-yl ) ethyl ketone 0.0491g, productive rate 98%.
[0042] 2,2,2-Trifluoro-1-((1,1'-biphenyl-4-yl)ethanone
[0043] white solid, 1 H NMR (500MHz, CDCl 3 )δ8.16(d, J=8.5Hz, 2H), 7.77(d, J=8.5 Hz, 2H), 7.65(d, J=8.0Hz, 2H), 7.52-7.49(m, 2H), 7.46- 7.44(m,1H); 13 C NMR (126MHz, CDCl 3 )δ180.24(q, J=35.0Hz), 148.37, 139.27, 130.89(q, J=1.8Hz), 129.27, 129.05, 128.72, 127.79, 127.51, 116.91(q, J=291.3Hz) ...
Embodiment 2
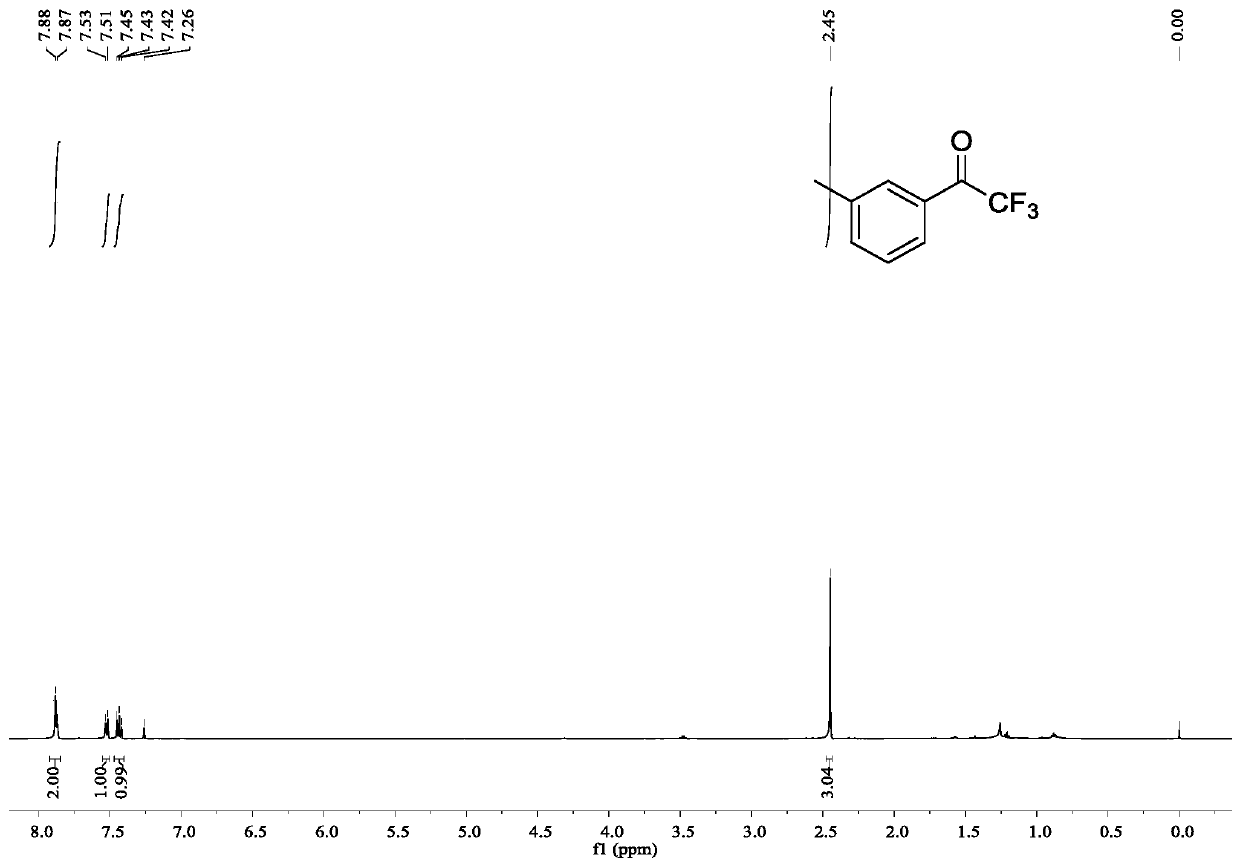
[0044] Example 2: Synthesis of 2,2,2-trifluoro-1-(3-tolyl)ethanone
[0045] The operation was the same as in Example 1, and 0.0252 g of 2,2,2-trifluoro-1-(3-methylphenyl)ethanone was obtained by reacting 3-methylphenylboronic acid with NTFTS, with a yield of 67%.
[0046] 2,2,2-Trifluoro-1-(3-methylphenyl)ethanone
[0047] colorless liquid, 1 H NMR (500MHz, CDCl 3 )δ7.88-7.87(m,2H),7.52(d,J=7.6Hz,1H),7.45-7.42(m,1H),2.45(s,3H); 13 C NMR (125MHz, CDCl 3 )δ180.80(q, J=34.8Hz), 139.25, 136.50, 130.61(q, J=1.8Hz), 130.09, 129.08, 127.52(q, J=2.1 Hz), 116.85(q, J=291.4Hz) ,21.45
Embodiment 3
[0048] Example 3: Synthesis of 2,2,2-trifluoro-1-((4-tert-butyl)phenyl)ethanone
[0049] In a 25mL reactor, tris(dibenzylideneacetone)dipalladium (0.0046g, 0.005mmol), tricyclohexylphosphine (0.0057g, 0.02mmol) and NTFTS (0.0687g, 0.2mmol), nitrogen replacement 3 times , 2 mL of anhydrous 1,4-dioxane was added, 4-tert-butylphenylboronic acid (0.0534 g, 0.3 mmol) was added with stirring, and stirred at 30° C. for 24 h. Column chromatography (silica gel, 200-300 mesh; developer, petroleum ether: ethyl acetate = 100:1) to obtain 2,2,2-trifluoro-1-((4-tert-butyl)phenyl)ethanone 0.0276g, 60% yield.
[0050] 2,2,2-Trifluoro-1-((4-tert-butyl)phenyl)ethanone
[0051] white solid, 1 H NMR (500MHz, CDCl 3 )δ8.02(d, J=7.9Hz, 2H), 7.56(d, J=8.6 Hz, 2H), 1.36(s, 9H); 13 C NMR (125MHz, CDCl 3 )δ180.24(q, J=34.8Hz), 159.97, 130.31(q, J=2.1Hz), 127.48, 126.27, 116.92(q, J=291.5Hz), 35.61, 31.04
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com