Use of a polypeptide in the preparation of drugs for preventing or treating metabolic syndrome
A metabolic syndrome and drug technology, applied in the field of biomedicine, can solve problems such as unsatisfactory curative effect, achieve the effects of reducing fat tissue weight, improving insulin resistance, and lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1. Preparation of polypeptide
[0038] Peptide Glu 1 -Thr 2 -Pro 3 -Asp 4 -Cys 5 -Phe 6 -Trp 7 -Lys 8 -Tyr 9 -Cys 10 -Val 11 (disulfide bond Cys 5 -Cys 10 ) is chemically synthesized (specifically by solid-phase synthesis), and the HPLC purity is greater than 95%. The peptide powder was dissolved in physiological saline to prepare a solution of 1000 μg / ml, and after aliquoting, it was frozen and stored at -80°C for later use. Dilute with normal saline to the corresponding concentration when used.
Embodiment 2
[0039] Embodiment 2. Effect of active polypeptide on normal animals
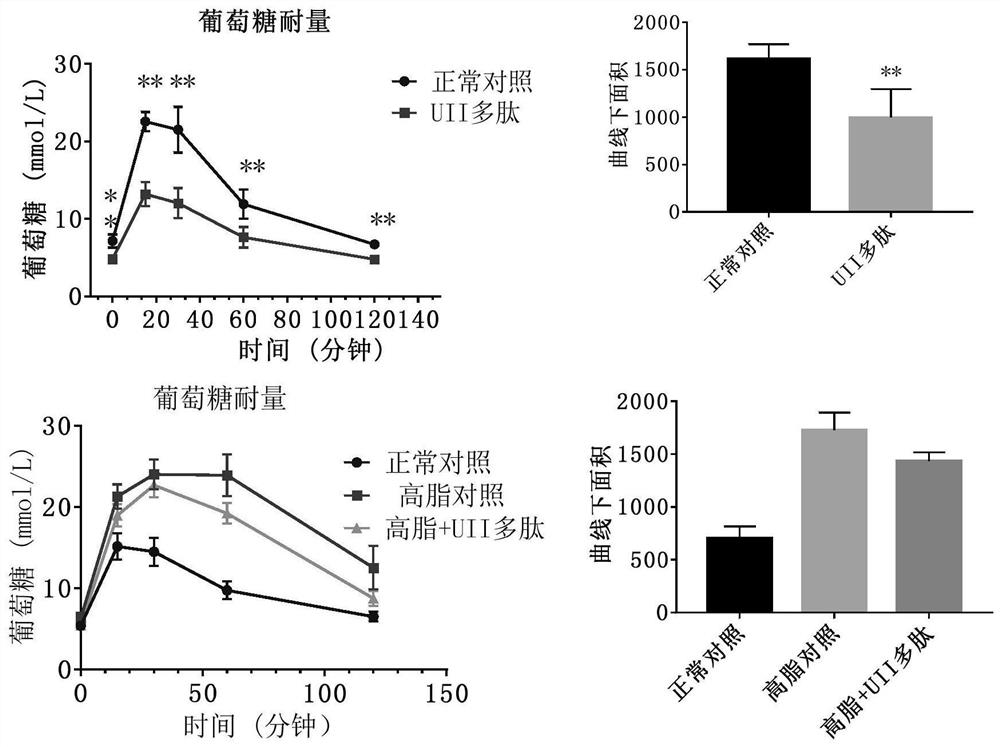
[0040] 10 C57BL / J male mice (Beijing Huafukang Biotechnology Co., Ltd.), weighing 17-20 g, were fed with complete nutritional feed, without restriction of water. Urotensin 27.8 μg / kg (20 nmol / kg) was administered, and body weight was monitored weekly. Once a day for 14 days. Animals were fasted overnight before glucose and insulin tolerance assays. 2g / kg or 0.75U / kg insulin were administered after measuring basal blood glucose. Blood glucose levels were measured at 15, 30, 60, and 120 minute time points. After the experiment, the visceral index and serum markers were measured.
Embodiment 3
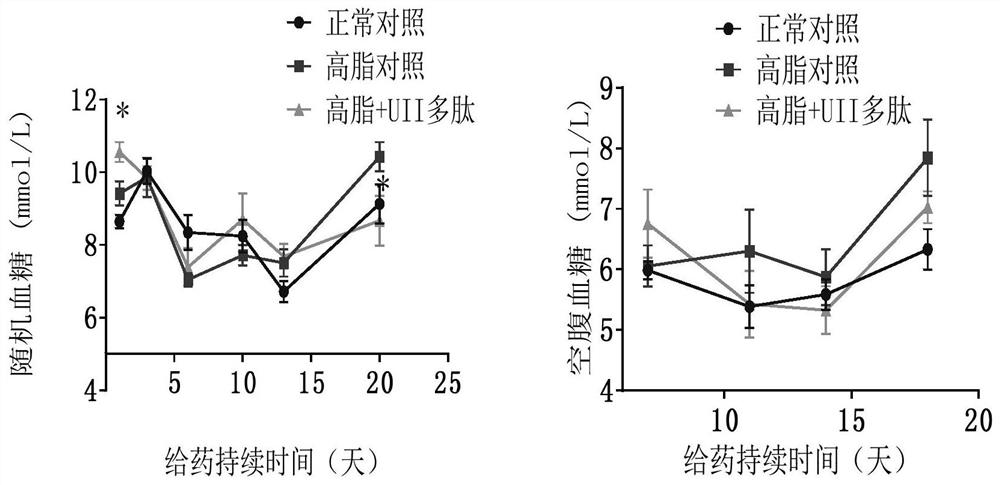
[0041] Example 3. Effect of Active Polypeptides on High Fat Feed Induced Metabolic Syndrome / Type 2 Diabetes / Obesity Animals
[0042] Male C57BL / J mice, weighing 17-20 g, were fed with high-fat diet and were not limited to water. C57BL / J mice of the same age were used as a normal control group, weighing 17-20 g, fed with complete nutrition feed, without water restriction. Monitor body weight weekly. After the high-fat-fed mice were 10 g heavier than the normal control, the mice in the obese group were randomly divided into two groups: the high-fat control group and the Urotensin 2 administration group. The urotensin administration group was intraperitoneally injected with polypeptide 27.8 μg / kg (20 nmol / kg), and the normal and high-fat control groups were intraperitoneally injected with the same volume of normal saline. Continuous administration for 3 weeks. Blood sugar and body weight were measured weekly. Animal deaths were recorded. After the experiment, the visceral in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com