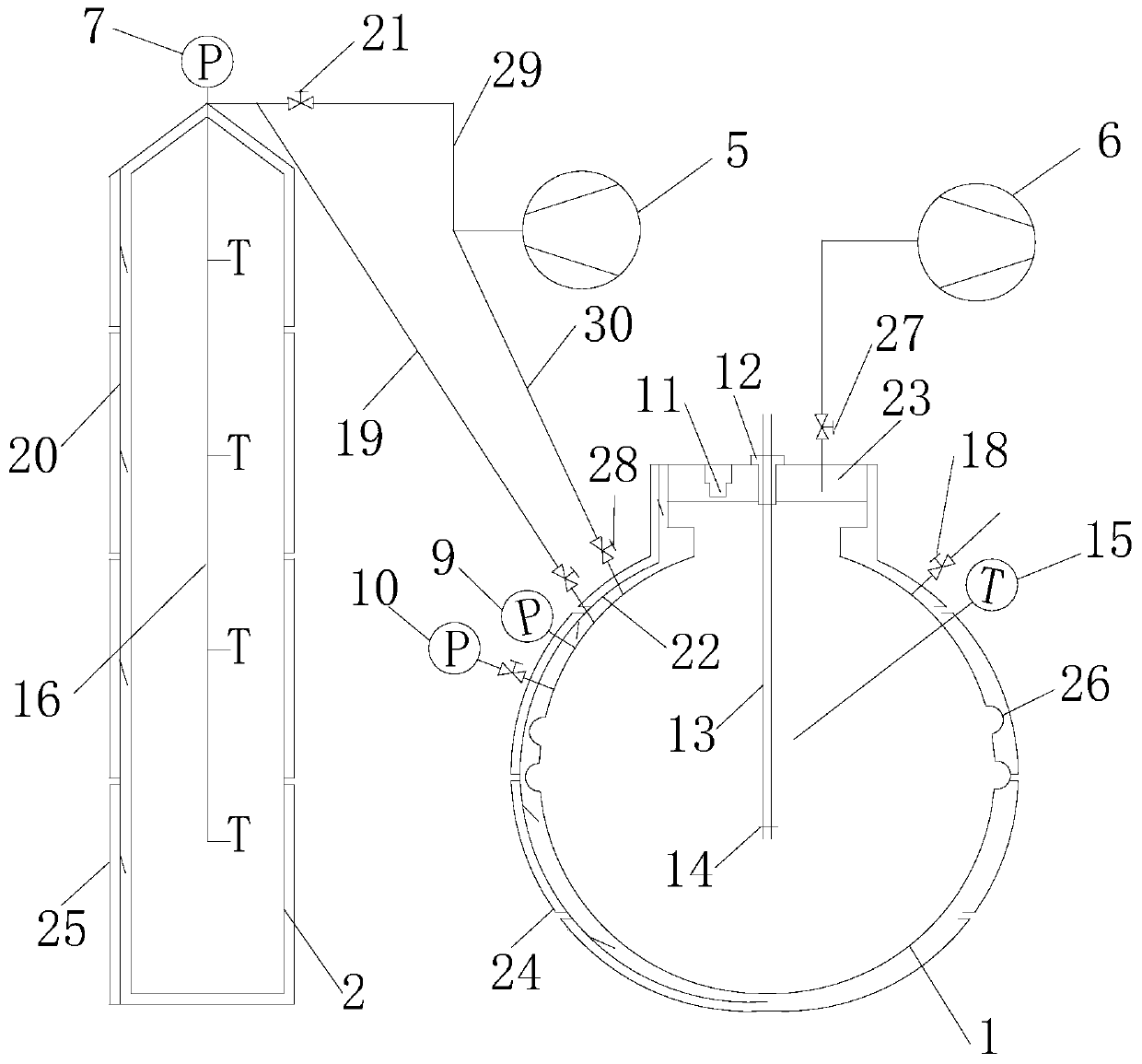
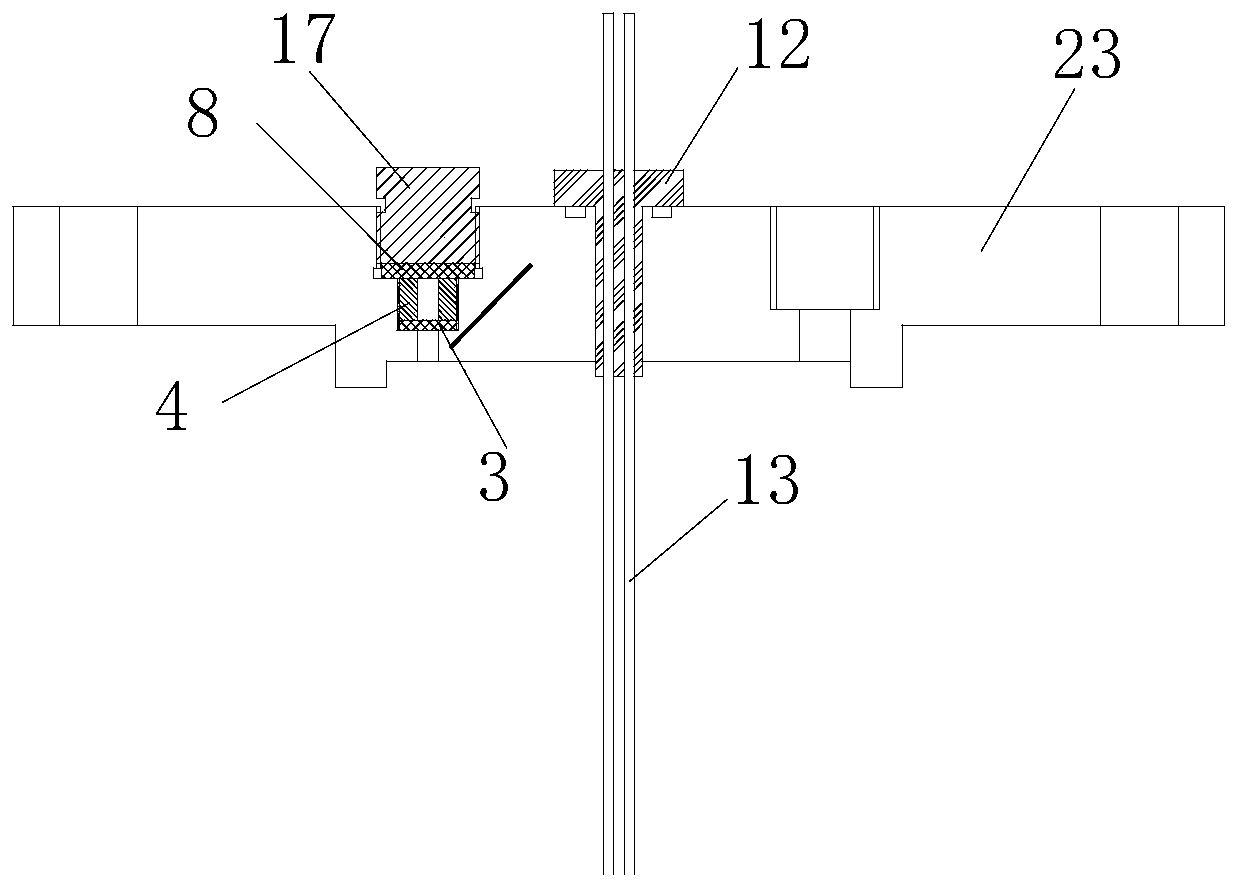
Measuring device for high-pressure explosion limit of flammable liquid and gas distribution method for mole fraction
A technology of explosion limit and measuring device, which is applied in the fields of material explosiveness, computer-aided design, design optimization/simulation, etc., and can solve the problem that the volume ratio cannot represent the actual ratio of flammable liquids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Use this device to prepare mixtures of n-pentane and air with different mole fractions under the test conditions of 30°C and 800KPa. image 3 Be the comparison of the mole fraction of n-pentane obtained according to the mole fraction gas distribution method of the present invention and the volume fraction of n-pentane obtained according to the ideal gas state equation, image 3 The absolute deviation and relative deviation of the mole fraction and the volume fraction are respectively given in the figure. It can be seen from the figure that when the mixed gas with a small mole fraction of n-pentane is prepared, the actually prepared mole fraction and volume fraction are very close. However, with the increase of the n-pentane mole fraction, the deviation between the mole fraction and the volume fraction is constantly increasing. When the n-pentane mole fraction reaches 20%, the relative deviation between the two has exceeded 20%, and it cannot be used at this time. Volume...
Embodiment 2
[0108] Using the device under the test conditions of 50° C. and under different initial pressure conditions, a mixture with an ethanol mole fraction of 18.5% (about the concentration of ethanol explosion upper limit) was prepared. Figure 4 It is the comparison of the mole fraction of ethanol obtained according to the mole fraction gas distribution method of the present invention and the volume fraction of ethanol obtained according to the ideal gas state equation, Figure 4 The absolute deviation and relative deviation of the mole fraction and the volume fraction are respectively given in the figure. It can be seen from the figure that when the initial pressure is small, the mole fraction and the volume fraction of ethanol actually prepared are very close. However, as the initial pressure increases As the pressure increases, the deviation between the molar fraction and volume fraction of ethanol obtained in actual preparation continues to increase. When the initial pressure is...
Embodiment 3
[0110] Using this device under the test condition of 100kPa, under different initial temperature conditions, the mixture with the toluene mole fraction of 7.0% (about toluene explosion limit concentration) was prepared. Figure 5 It is the comparison of the mole fraction of toluene obtained according to the mole fraction gas distribution method of the present invention and the volume fraction of toluene obtained according to the ideal gas state equation, and the absolute deviation and relative deviation of the mole fraction and volume fraction are respectively provided in the figure ,from Figure 5 It can be seen from the figure that when the initial temperature is high, the actual prepared toluene mole fraction and volume fraction are very close, but as the initial temperature decreases, the deviation between the actually prepared toluene mole fraction and the volume fraction increases continuously , when the initial temperature is 0°C, the relative deviation between the two ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com