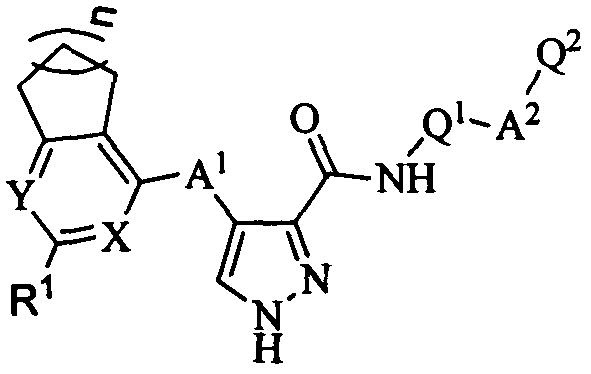
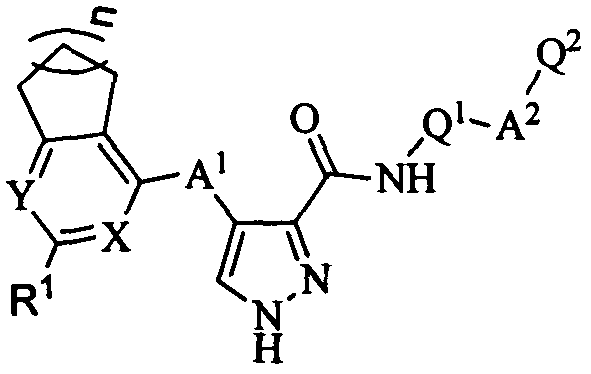
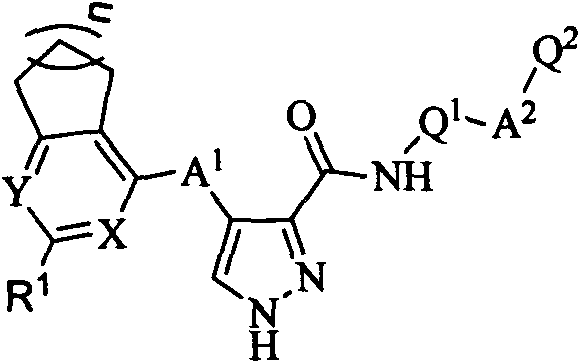
4-(saturated aliphatic ring-pyrimidine/pyridine substituted)amino-1H-3-pyrazol carboxamide FMS-like tyrosine kinase 3 (FLT3) inhibitor and application thereof
A technology of halogenated alkyl and alkyl, which is applied in the field of new FLT3 kinase inhibitor compounds, and can solve the problems that there are no selective FLT3 inhibitors on the market.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] 1-Methyl-4-(4-nitrobenzyl)piperazine (I-a)
[0109]Add 10 g (46.3 mmol) of p-nitrobenzyl bromide and 100 mL of dichloromethane into a 500 mL single-necked bottle, and slowly add 4.7 g (47.0 mmol) of N-methylpiperazine (47.0 mmol) and three A mixture of 7.1 g (70.3 mmol) of ethylamine (70.3 mmol) in 20 mL of dichloromethane was heated to reflux for 1 h after the addition, and the starting material disappeared as detected by TLC (ethyl acetate:petroleum ether=1:2). Add 150 mL of chloroform and 100 mL of saturated sodium bicarbonate solution into the reaction liquid, and vigorously stir at room temperature for 30 min. The reaction solution was extracted with chloroform (100 mL×3), and the combined organic layers were washed once with water and saturated sodium chloride (100 mL×1). Dry over anhydrous magnesium sulfate, filter, and distill off the solvent under reduced pressure to obtain 8.5 g of a light yellow solid with a yield of 78.1%. The product does not need further ...
Embodiment 2
[0111] 4-((4-Methylpiperazin-1-yl)methyl)aniline (I-b)
[0112] Add I-a crude product 8.5g (36.2mmol), FeO(OH) / C catalyst 2.0g and 95% ethanol 100mL in 500mL single-necked bottle, heat to reflux, slowly add dropwise the mixed solution of hydrazine hydrate 25mL and 95% ethanol 20mL, TLC The disappearance of the starting material was detected (methanol:chloroform=1:15). After hot suction filtration, the filter cake was washed twice with hot ethanol (30 mL×2), and the solvent was evaporated under reduced pressure to obtain a white solid, which was dried in vacuo to obtain (I-b) 6.7 g, yield 90.3%. The product was directly put into the next reaction without further purification. 1 H NMR (300MHz, DMSO) δ8.1(d, J=8.5Hz, 2H, ArH), 7.5(d, J=8.5Hz, 2H, ArH), 4.0(s, 2H, -NH 2 ), 3.5(s, 2H, -CH 2 -), 2.3-2.5 (br, 8H, -CH 2 -×4), 2.1(s, 3H, -CH 3 )
Embodiment 3
[0114] N-(4-((4-methylpiperazin-1-yl)methyl)phenyl-4-nitro-1H-pyrazole-3-carboxamide (I-c)
[0115] In a 250mL round bottom flask, add 7.5g (36.6mmol) of I-a' crude product, 6.3g (40.1mmol) of 4-nitro-1H-pyrazole-3-carboxylic acid, 8.4g (44.0mmol) of EDC·HCl, HOBt 6.0g (44.4mmol) and anhydrous DMF100mL, stirred at room temperature for 24h. The disappearance of the starting material was detected by TLC (methanol:chloroform=1:10). The reaction solution was poured into 200 mL of ice water, a large amount of light yellow solid was precipitated, left to stand, and filtered to obtain a yellow solid, the obtained crude product was recrystallized with a mixed solvent of ethyl acetate and methanol to obtain (I-e) 11.1 g, yield 88.2%. MS[M+H] + 345.3. 1 H NMR (300MHz, DMSO) δ14.2(s, 1H, -NH-, Pyrazole), 10.6(s, 1H, -NHCO-), 8.8(s, 1H, ArH), 7.6(d, J=8.7Hz , 2H, ArH), 7.3 (d, J=8.7Hz, 2H, ArH), 3.4 (s, 2H, -CH 2 -), 2.3-2.4 (br, 8H, -CH 2 -×4), 2.2(s, 3H, -CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com