Simple preparation method of Cu2O micropowder
A micro-powder, simple technology, applied in the direction of copper oxide/copper hydroxide, etc., can solve the problems of high price, production pollution, high toxicity, etc., and achieve the effect of low cost, large product volume and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] a Cu 2 The simple and easy preparation method of O micropowder comprises the following steps:
[0017] (1) CuCl 2 2H 2 Add O and lactose to deionized water to make a solution, and then add NaOH solid to the above solution to obtain an alkaline reaction precursor;
[0018] (2) Put the prepared precursor solution above at room temperature for oxidation-reduction reaction to obtain Cu 2 O micropowder.
[0019] Lactose and CuCl described in step (1) 2 2H 2 The mass ratio of O is 2:1.
[0020] The quality of NaOH described in step (1) is 0.1-0.7g.
[0021] The volume of the reaction precursor solution described in step (1) is 120ml.
[0022] The reaction temperature of the oxidation-reduction reaction in step (2) is 25°C, and the reaction time is 3-12h.
Embodiment 1
[0024] a Cu 2 The simple and easy preparation method of O micropowder comprises the following steps:
[0025] (1) 1.7gCuCl 2 2H 2 O and 3.4g of lactose are added into 120ml of deionized water to form a solution, and the prepared solution is divided into four equal parts, and 0.1g, 0.3g, 0.5g and 0.7g of NaOH solid are added respectively to four parts of the solution to obtain four parts of alkaline Reaction precursor;
[0026] (2) Put the prepared precursor solution above at room temperature for oxidation-reduction reaction to obtain Cu 2 O micropowder.
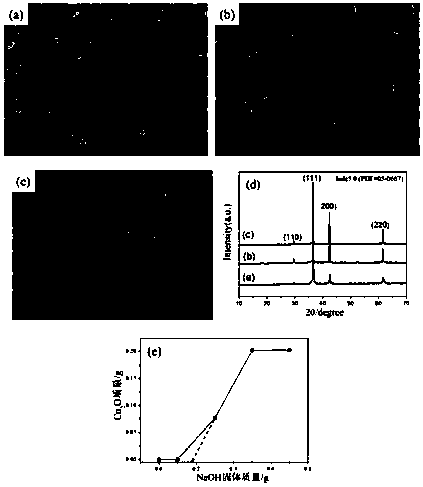
[0027] After preliminary analysis of the product generated by the reaction with SEM and XRD, it can be seen that with the increase of the amount of NaOH, the product Cu 2 O powder particles gradually transform from random particles to quasi-cubes, with a size of about 1.5um (such as figure 1 a~ figure 1 shown in c). The XRD spectrum of the product is as figure 1 As shown, by combining with bulk Cu 2 After comparing ...
Embodiment 2
[0030] a Cu 2 The simple and easy preparation method of O micropowder comprises the following steps:
[0031] (1) CuCl 2 2H 2 Add O and lactose to deionized water to make a solution, and then add NaOH solid to the above solution to obtain an alkaline reaction precursor;
[0032] (2) Put the prepared precursor solution above at room temperature for oxidation-reduction reaction to obtain Cu 2 O micropowder.
[0033] When lactose is not added to the reaction system, there is no Cu in the reaction system 2 The O product appeared, and with the increase of the amount of lactose, a brick-red precipitate gradually appeared in the reaction system. Depend on figure 2 a~ figure 2 c It can be seen that with the increase of the amount of lactose (0.1g, 0.3g, 0.5g), the generated Cu 2 The morphology of O powder gradually changed from regular spherical particles to irregular particles, and its size gradually decreased.
[0034] Based on the above experimental results, it can be co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com