Serum miRNA marker and application of serum miRNA marker in early diagnosis for pancreatic cancer induced by pancreatitis
A marker and serum technology, applied in the field of molecular biology, can solve the problems of poor stability and repeatability of chip technology information quality, high detection cost, and high miRNA purity requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, embodiment 2, embodiment column 3 and embodiment 4 provide a kind of miRNA detection kit, comprise following components:
[0033]
[0034]
[0035] The composition of the above kit can be used for 100 times of fluorescent quantitative PCR reactions and stored at -20°C. The sources of the above reagents are provided by the above reagent companies.
[0036] The present invention provides the application of the miRNA detection kit in the early diagnosis of pancreatic cancer mediated by pancreatitis, and the specific implementation method is as follows:
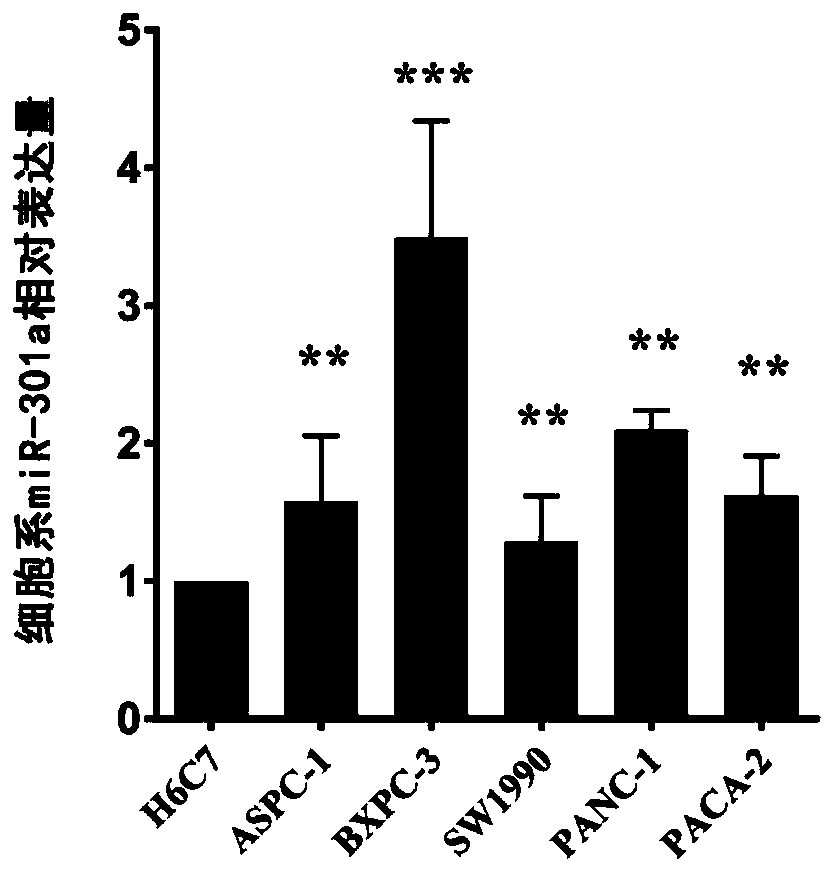
[0037] Example 1: Detection of expression levels of miR-301a in pancreatitis, pancreatic cancer patient plasma, pancreatic cancer tissues and cell lines using the above kit
[0038] 1. Specimen collection
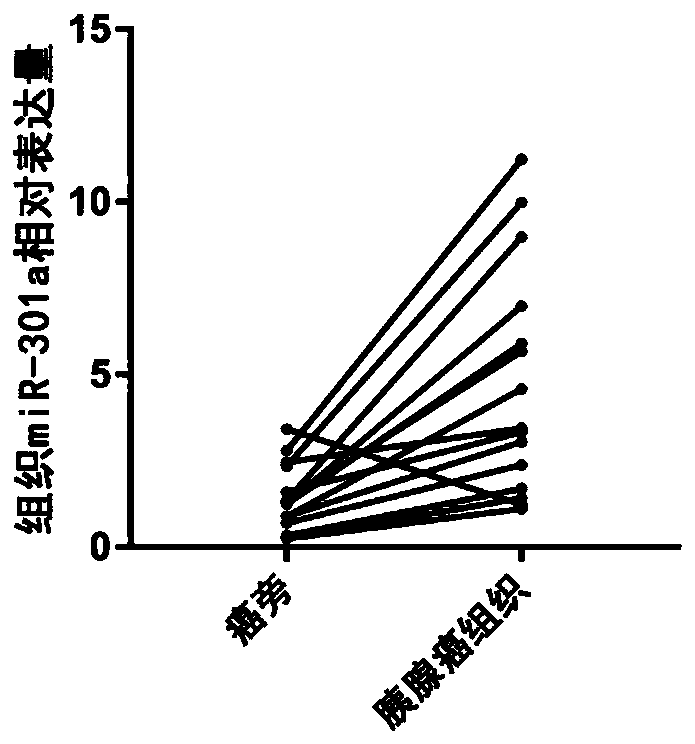
[0039] Plasma samples were collected from 70 non-small cell pancreatic cancer patients with a history of pancreatitis who were clinically confirmed and had not received any treatment. Plasma sample...
Embodiment 2、 Embodiment 3、 Embodiment 4
[0078] Example 2, Example 3, Example 4: Using the above kit to detect the expression level of miR-301a in the plasma of patients with pancreatic cancer
[0079] A kit for the early diagnosis of inflammation-mediated pancreatic cancer provided in Example 2, Example 3, and Example 4 differs from Example 1 in that 10 μM miRNA in the reverse transcription (RT-PCR) reaction system reverses the The volume ratios of primers, 10 μM U6 reverse transcription primer, nuclease-free water, 5× reverse transcription buffer and enzyme preparation are different, see Table 2:
[0080] Reagent volume in table 2 reverse transcription reaction system
[0081]
[0082] Quantitative PCR detection of plasma miR-301a expression in 63 patients with pancreatic cancer, see Figure 6 The volume ratio of the 10 μM miRNA reverse transcription primer, 10 μM U6 reverse transcription primer, nuclease-free water, 5× reverse transcription buffer, and enzyme preparation used in Example 1 was 1:1:16.75:5:1.25,...
Embodiment 5
[0083] Example 5: Correlation analysis of plasma miR-301a level in patients with pancreatic cancer and pancreatitis-related factors IL-6, TNF-α
[0084] 1. Analysis method: SPSS 19.0 software was used to conduct Pearson correlation test to analyze the correlation between plasma miR-301a level and pancreatitis markers.
[0085] 2. Experimental results: analysis of the correlation between plasma miR-301a level and common pancreatitis markers IL-6 and TNF-α, see Figure 7 , Figure 8 , the results of Pearson correlation test analysis showed that the correlation coefficients R of plasma miR-301a, IL-6 and TNF-α in patients with pancreatic cancer were 0.35 and 0.65, respectively, P<0.01, indicating that the plasma miR-301a level and IL-6 -6 and TNF-α have a certain correlation, further indicating that the level of plasma miR-301a has a certain correlation with the well-known carcinoembryonic antigen CEA and pancreatic cancer marker CA199, circulating miR-301a is expected to play a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com