Preparation method and application of coumarin glycoside compounds
A technology of coumarin glycosides and compounds, applied in the field of pesticides, to achieve significant economic and social benefits and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
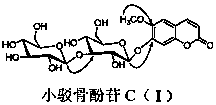
[0012] In the specific implementation of the present invention, a kind of coumarin glycoside compound, the compound is made of 20kg of small bark bone as a raw material, and is extracted 3 times by heating at 90°C with ethanol with 2-5 times the weight of the raw material and a volume concentration of 95%. The extraction time is 1 hour, and ethanol is recovered under reduced pressure to obtain extract-like ethanol extract, which is suspended in 4.8L of distilled water, and extracted 3 times with petroleum ether, ethyl acetate, and n-butanol successively, 4.8L each time, The time is 1.5 hours; the n-butanol extraction part is separated by AB-8 macroporous adsorption resin column chromatography, and the volume ratio is 0:100, 30:70, 95:5 ethanol-water mixed solvent for gradient elution, each One gradient uses 5.0L eluent, the flow rate is 10mLmin -1 , the water eluent was discarded, and the eluate fractions with a volume ratio of 30:70 and 95:5 ethanol-water were collected respe...
Embodiment 2
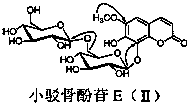
[0014] In the specific implementation of the present invention, a kind of coumarin glycoside compound, with 15kg of small barb bone as raw material, is 3 times of raw material weight, and volume concentration is 75% ethanol heating 95 ℃ of reflux extraction 3 times, and each extraction time is For 1 hour, ethanol was recovered under reduced pressure to obtain extract-like ethanol extract, which was suspended in 3.6L of distilled water, and extracted three times with petroleum ether, ethyl acetate, and n-butanol in sequence, 3.6L each time, for 1.5 hours ; The n-butanol extraction part is separated by AB-8 macroporous adsorption resin column chromatography, and the volume ratio is 0:100, 30:70, 95:5 ethanol-water mixed solvent for gradient elution, and each gradient uses 3.8 L eluent, the flow rate is 5mLmin -1 , the water eluent was discarded, and the eluate fractions with a volume ratio of 30:70 and 95:5 ethanol-water were collected respectively, concentrated and dried under ...
Embodiment 3
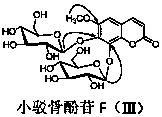
[0016] In the specific implementation of the present invention, a kind of coumarin glucoside compound, with 18kg of small barb bone as raw material, with 4 times of raw material weight, volume concentration is 85% ethanol heating 92 ℃ of reflux extractions 3 times, each extraction time is After 1 hour, ethanol was recovered under reduced pressure to obtain extract-like ethanol extract, which was suspended in 4.5L of distilled water, and extracted three times with petroleum ether, ethyl acetate, and n-butanol in sequence, 4.5L each time, for 1.5 hours ; The n-butanol extraction part is separated by AB-8 macroporous adsorption resin column chromatography, and the volume ratio is 0:100, 30:70, 95:5 ethanol-water mixed solvent for gradient elution, and each gradient uses 4.5 L eluent, the flow rate is 8mLmin -1 , the water eluent was discarded, and the eluate fractions with a volume ratio of 30:70 and 95:5 ethanol-water were collected respectively, concentrated and dried under red...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com