Preparation method, purification method and applications of PEG-modified recombinant humanized urate oxidase
A technology of urate oxidase and urate oxidase protein, which is applied in the field of protein engineering and can solve problems such as sensitivity, loss of enzyme activity, and intolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Construction of humanized urate oxidase high-expression strain
[0077] The sequence (SEQ ID NO :3), after adding NcoI (CCATGG) and XhoI (CTCGAG) double restriction sites at the front and rear ends of SEQ ID NO: 3, artificially synthesized SEQ ID NO: 4 (for Suzhou Jinweizhi Biotechnology Co., Ltd.), after synthesis The sequence of SEQ ID NO: 4 was double digested (NcoI and XhoI) and connected to the same double digested pET-28a vector, and then transformed into DH5α cells to amplify and extract the plasmid to obtain an expression vector.
[0078] SEQ ID NO:4
[0079]CCATGGATTATAAGAAAAATGATGAAGTGGAGTTTGTGCGCACCGGCTATGGCAAGGAAATGGTGAAGGTGCTGCACATCCAGCGTGATGGCAAATATCATAGCATTAAAGAAGTGGCCACCAGCGTGCAGCTGACCCTGAGCAGCAAAAAGGATTACCTGCACGGCGACAACAGCGATATCATTCCGACCGACACCATCAAGAATACCGTGCATGTGCTGGCCAAATTCAAGGGCATCAAGAGCATCGAGGCCTTCGCCATGAATATCTGCGAGCATTTTCTGAGCAGCTTCAACCACGTGATTCGTGCCCAGGTGTATGTGGAAGAAGTGCCGTGGAAGCGCTTCGAGAAAAATGGCGTGAAGCACGTGCATGCCTTTATCCATACCCCGACCGGCACCCACTTTTGC...
Embodiment 2
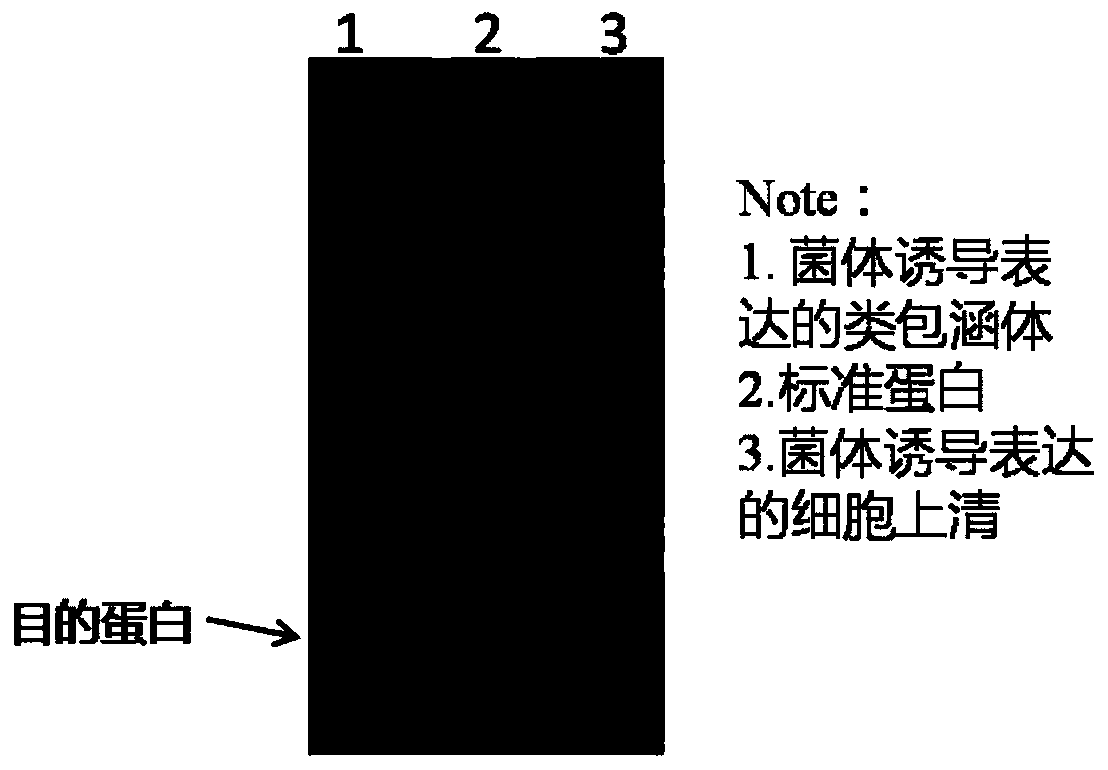
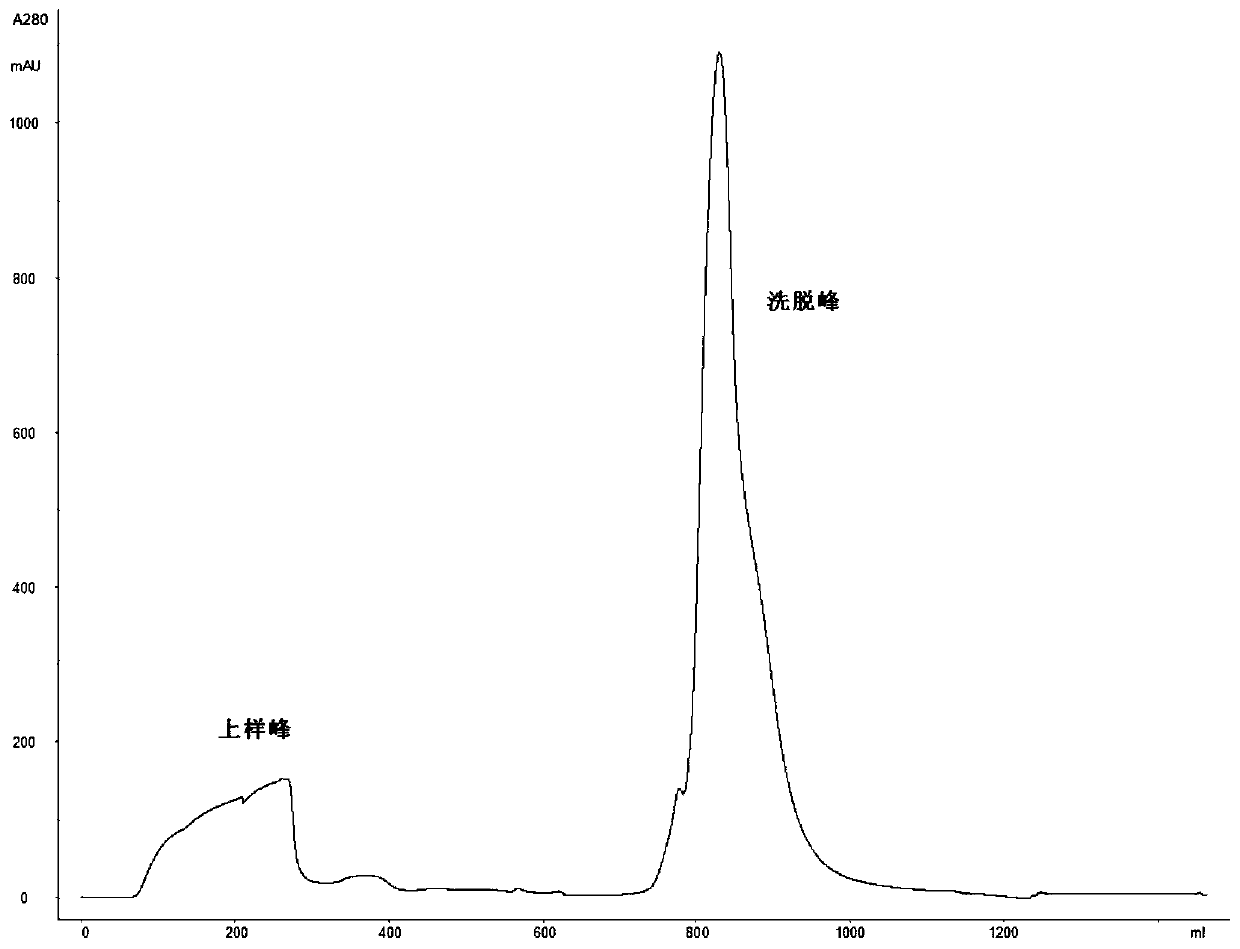
[0082] Expression and purification of humanized urate oxidase
[0083] Step (1). Protein expression:
[0084] a) Recovery of the target strain: take out the target bacteria frozen in glycerol from -80°C, dilute 100,000-fold with LB in a clean bench, spread it on an LB plate with 50 μg of Kanamycin, place it upside down in a 37°C incubator, and cultivate overnight Store in a refrigerator at 4°C and recover after 2 months.
[0085] b) Seed culture: Prepare 70ml of 50μg Kanamycin LB sterile medium in a 200ml triangular medicine bottle, pick a single colony on the plate and place it in the medium, and culture it overnight in a shaker at 37°C and 180rpm.
[0086] c) Batch culture and induced expression: Prepare 400ml of 50μg Kanamycin LB sterile medium in a 1L Erlenmeyer shaker flask, add 4ml of seed solution to the 400ml medium in an ultra-clean workbench (shake well before absorbing), seal and place in Cultivate in a shaker at 37°C and 250 rpm. Waiting for OD 600 At 0.6-0.8 (...
Embodiment 3
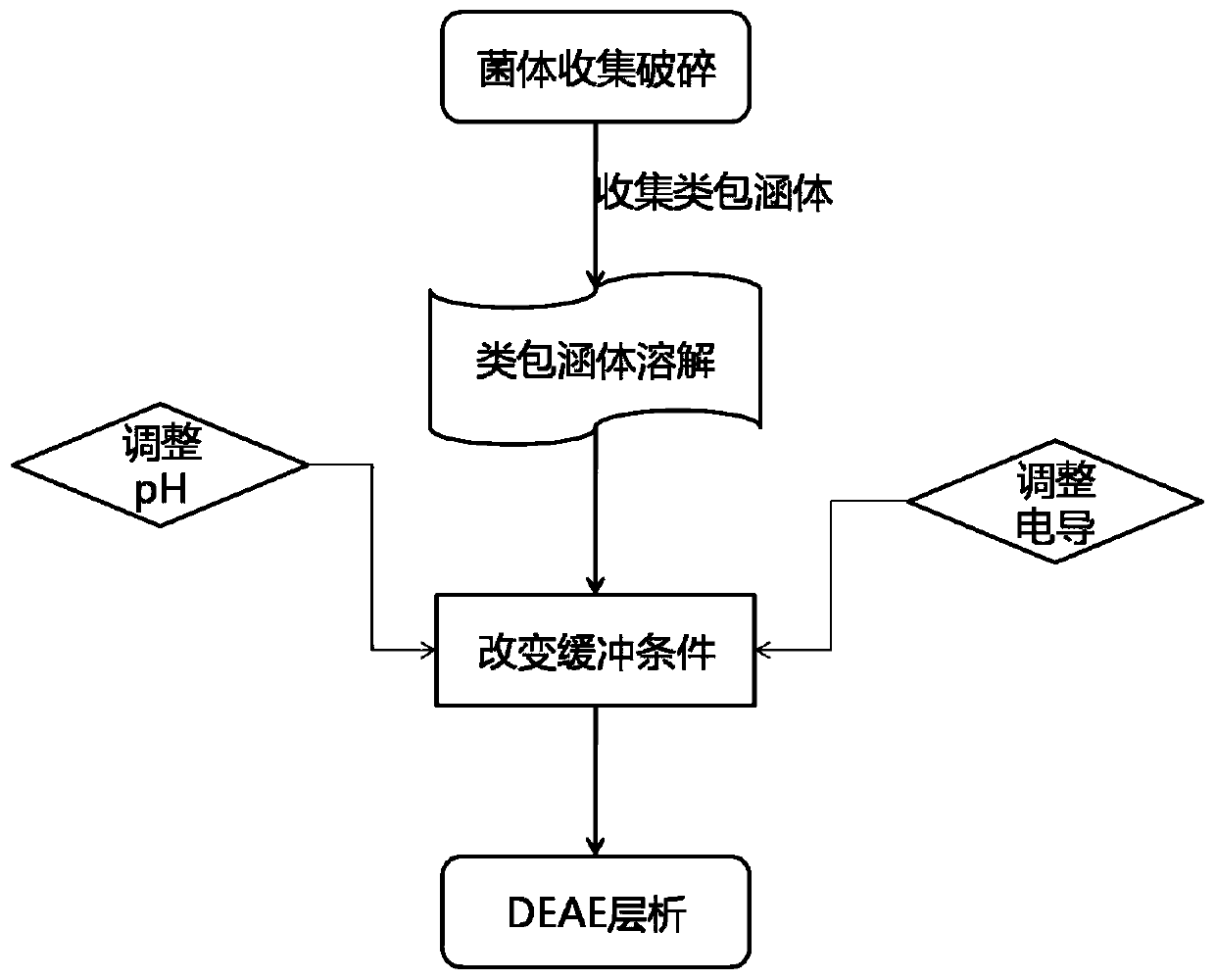
[0106] Step (4) Add 10% sucrose to the buffer solution, then add CH 3 COOH reduces the pH of the buffer to 8.5, and the rest of the steps are exactly the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com