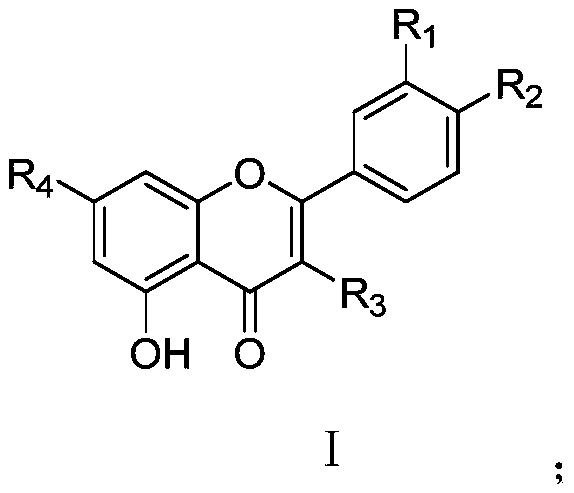
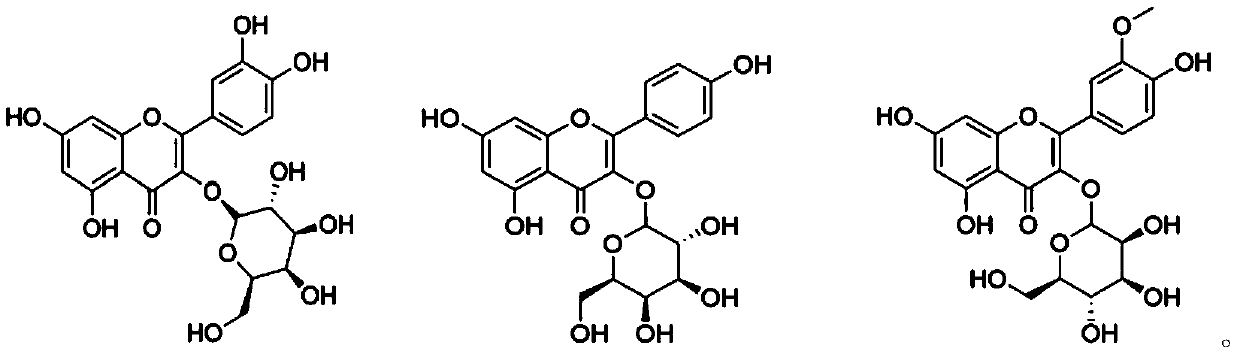
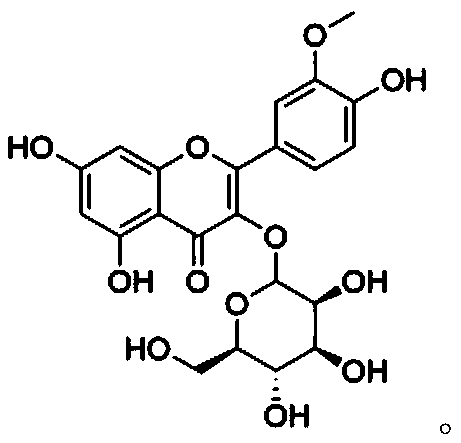
Application of flavone oxygen glycoside compounds in preparations of bacterial quorum sensing inhibitory drugs
A flavonoid oxoside and quorum sensing technology, applied in the field of biomedicine, can solve problems such as bacterial quorum sensing systems that have not yet been discovered, and achieve the effects of not easy bacterial resistance and great citation prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The biofilm inhibition experiment of embodiment 1 flavonoid oxyglycosides
[0031] Test compound: with furanone compound (Z)-4-bromo-5-(bromomethylene)-2(5H)-furanone as positive control, DMSO as negative control, positive drug and compound 1 of the present invention ~3 flavonoid oxyglycosides were formulated to 32, 64, 128 μg / mL, respectively.
[0032] Test strains: Pseudomonas aeruginosa 9027 (ATCC 9027), Pseudomonas aeruginosa 27853 (ATCC 27853) and drug-resistant Pseudomonas aeruginosa (drug-resistant strain).
[0033] Experimental method: Add 100 μL of the prepared compound to be tested to the well plate, and inoculate 100 μL of bacterial solution. A blank control group (200 μL of LB medium) and a negative control group (100 μL of each of LB medium and bacterial solution) were set up. Incubated in a 37°C incubator. After 20 hours, absorb the bacterial liquid on the surface layer of the hole, wash with distilled water three times, and wash away the planktonic bac...
Embodiment 2
[0037] Embodiment 2 anti-pseudomonas aeruginosa granules
[0038] Compound 1 10mg;
[0039] Lactose 15mg;
[0041] The preparation steps of anti-pseudomonas aeruginosa granules are:
[0042] (1) Take 10 mg of compound 1 in Example 1, first mix it with lactose for 10-15 minutes;
[0043] (2) Add 10% starch slurry to make soft material, pass through a 14-mesh sieve, and dry after granulating;
[0044] (3) Pass through a 12-mesh sieve, granulate and then dry to obtain anti-Pseudomonas aeruginosa granules.
Embodiment 3
[0045] Embodiment 3 anti-pseudomonas aeruginosa capsules
[0046]
[0047]
[0048] The preparation steps of anti-pseudomonas aeruginosa capsules are:
[0049] (1) Take 10 mg of compound 2 in Example 1, first mix it with lactose for 10-15 minutes;
[0050] (2) Add microcrystalline cellulose and mix for 10-15 minutes;
[0051] (3) Add talcum powder and mix for 3-5 minutes;
[0052] (4) The mixture is packed into a gelatin capsule shell to obtain an anti-Pseudomonas aeruginosa capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com