Application of dipterin b protein and/or dipterin b gene and antiviral drugs
An antiviral drug and B protein technology, which is applied in the field of biomedicine to achieve wide application prospects and the effect of inhibiting virus replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Further, in the embodiment of the present invention, the preparation method of the dipteropeptide B protein comprises:
[0026] S01: construct a recombinant plasmid containing the sequence shown in SEQ ID NO.2;
[0027] S02: The recombinant plasmid is transferred into a host cell for expression to obtain the dipteropeptide B protein.
[0028] In the embodiment of the present invention, when verifying the function of dipteropeptide B protein, the function verification can be achieved by constructing a recombinant plasmid that does not have a partial nucleotide sequence expressing the signal peptide in SEQ ID NO. 2. The specific steps include:
[0029] S011: extract the total RNA of Drosophila, and then reverse-transcribe to obtain cDNA;
[0030] S012: Amplify the nucleotide sequence of the non-signal peptide part in SEQ ID NO.2 from the cDNA;
[0031] S013: Connect the nucleotide sequence of the non-signal peptide part in SEQ ID NO. 2 with the backbone plasmid vector t...
Embodiment 1
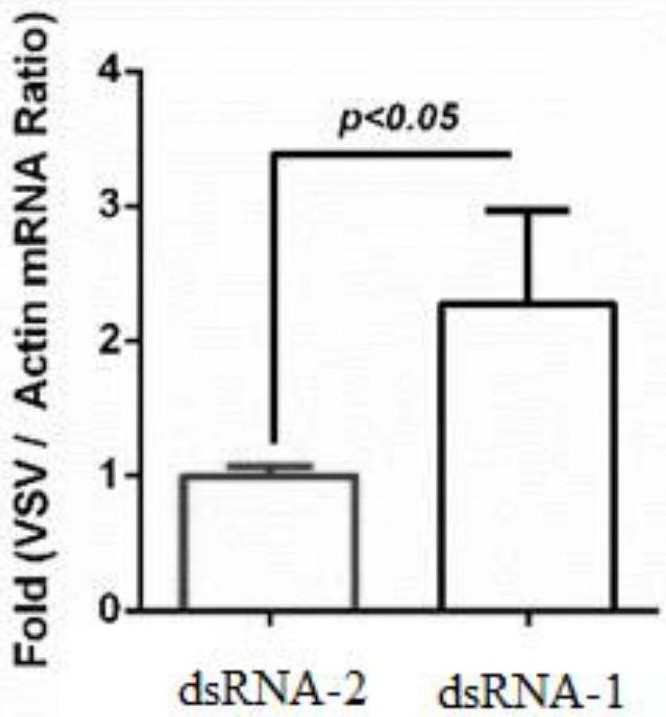
[0042] Example 1 Validation of dsRNA inhibiting DmDptB gene expression to promote replication of vesicular stomatitis virus
[0043] 1. Preparation of dsRNA
[0044] 1. The primers designed to prepare the coding DNA of the dsRNA that inhibits the expression of the DmDptB gene are as follows:
[0045] DmDptB-F (SEQ ID NO. 3):
[0046]
[0047] DmDptB-R (SEQ ID NO. 4):
[0048]
[0049] In the above primers, the region marked by the box is the T7 promoter sequence.
[0050] 2. The total RNA of S2 cells was extracted and reverse transcribed into cDNA. Using cDNA as a template, PCR amplification was performed with a primer pair composed of DmDptB-F and DmDptB-R to obtain a PCR amplification product.
[0051] 3. Take the PCR amplification product obtained in step 2, use Ambion MEGAscriptT7High YieldTranscription Kit (catalog No. AM1334) and conduct in vitro transcription according to the kit instructions. Since both DmDptB-F and DmDptB-R have T7 promoters, in vitro transcr...
Embodiment 2
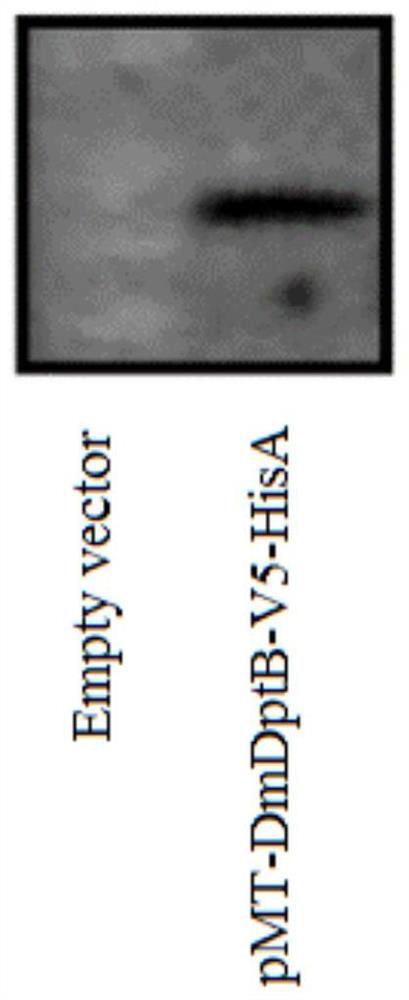
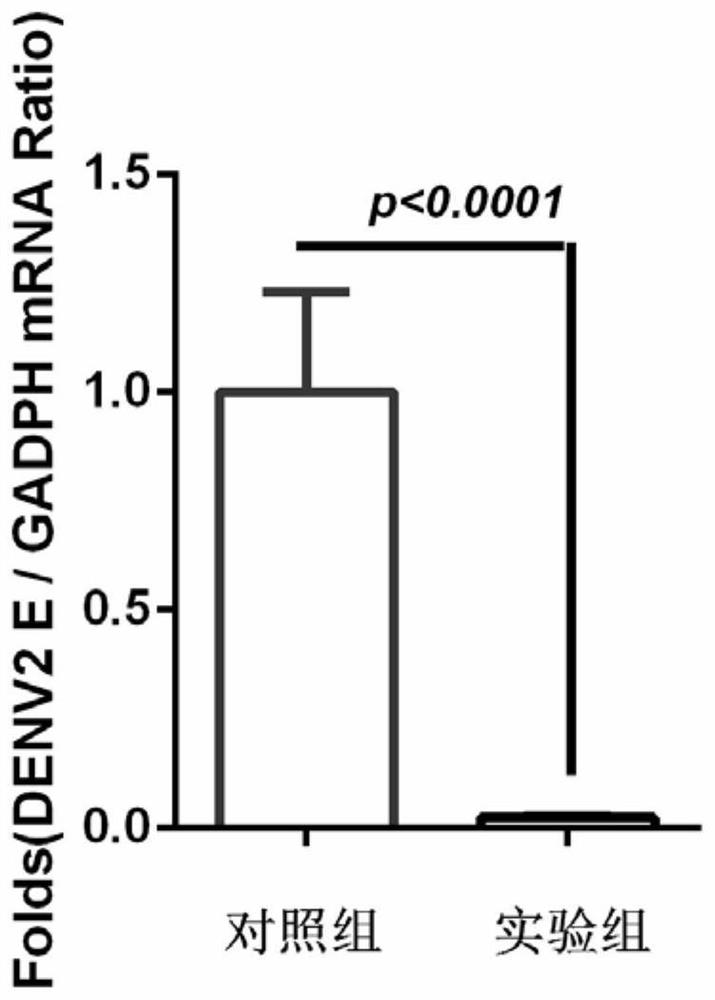
[0073] Example 2 Validation of DmDptB protein in inhibiting dengue virus replication
[0074] 1. Construction of recombinant plasmid pMT-DmDptB-V5-HisA
[0075] 1. The total RNA of Drosophila was extracted and reverse transcribed to obtain cDNA.
[0076] 2. Using the cDNA obtained in step 1 as a template, use F1 and R1 primer pairs to carry out PCR amplification to obtain a PCR amplification product. The primer sequences are as follows (the underlined sequences represent restriction sites):
[0077] F1 (SEQ ID NO. 12): 5'-AAGAA gaattc TTATCCCTATCCTGATCCCCG-3’
[0078] F1 (SEQ ID NO. 13): 5'-AAGAA tctaga AAACCTGAAGGTATACACTC-3’
[0079] 3. The PCR amplification product of step 2 was double digested with restriction enzymes EcoRI and XbaI, and the digested product was recovered.
[0080] 4. The plasmid pMT / BiP / V5-HisA was digested with restriction enzymes ECORI and XbaI, and the vector backbone of about 5000 bp was recovered.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com