Polybenzyl derivative and its pharmaceutical composition, its preparation method and its application
A compound, Gastrodia elata technology, applied in the preparation of sugar derivatives, drug combinations, preparation of organic compounds and other directions, can solve the problems of reports, no biological activity, no reports of polybenzyl derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Compound 1-6 separation and purification:
[0027] Partial extraction and separation from ethyl acetate of Gastrodia elata. Fresh Gastrodia elata (45.0kg) slices were soaked in 90% ethanol for three times, filtered, the residue was soaked in 50% ethanol for three times and filtered, the residue was boiled once, all the filtrates were combined, and concentrated under reduced pressure until alcohol-free. Extract with ethyl acetate 3 times, obtain ethyl acetate extraction part (102g), water phase is through macroporous resin, water, 50% ethanol and 90% elution respectively, obtain water part (1.6kg), 50% ethanol (71g ), 90% (8g). Mix 88g of sample with 88g of silica gel, and use 880g of silica gel for wet packing. with EtOAc:CHCl 3 The system was eluted at 0:100, 5:95, 10:90, 20:80, 40:60, and MeOH, and 5 fractions were obtained. Guided by activity tracking, the active part and the inactive part were separated and purified, as shown in the table 2 is the test results o...
Embodiment 2
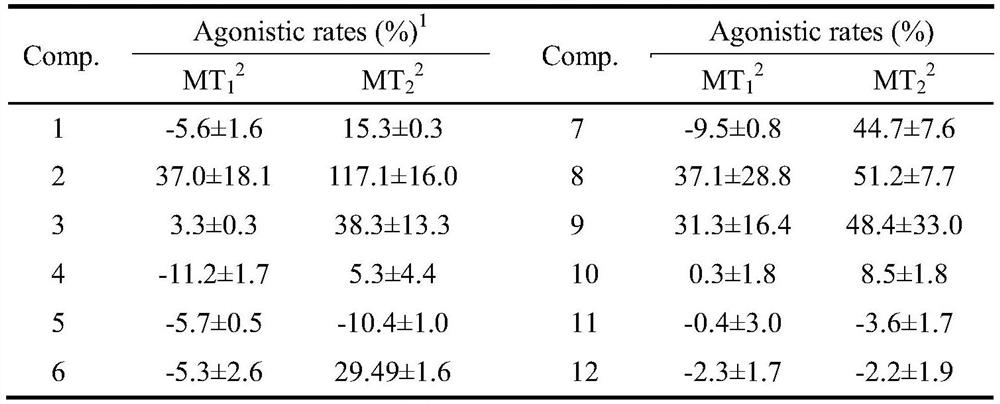
[0060] Compounds 1-12 on melatonin receptor MT 1 and MT 2 Agonistic activity of the receptor.
[0061] 1 Materials and methods
[0062] 1.1 Materials:
[0063] melatonin receptor MT 1 and MT 2 The cell lines used for agonistic activity screening correspond to human kidney epithelial cells HEK293-MT 1 and HEK293-MT 2 ; Cell culture medium (Dulbecco's Modified Eagle Medium, DMEM) containing 10% fetal bovine serum; No-wash calcium flow kit.
[0064] 1.2 Instrument: CO 2 Constant temperature incubator Thermo Forma 3310 (USA); Inverted biological microscope XD-101 (Nanjing); Flexstation 3 Benchtop Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, California, USA).
[0065] 1.3 Experimental process
[0066] Coat the 96-well black-walled transparent-bottom cell culture plate with the substrate BD Matrigel, put it in a constant temperature incubator at 37°C for 1 hour, absorb the supernatant, and dilute it with 4×10 4 Density per well, the corresponding HEK293 cell...
preparation Embodiment
[0076] 1. Compound 1-12 was prepared according to the method of Example 1. After dissolving with a small amount of DMSO, water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
[0077] 2. Compound 1-12 was prepared according to the method of Example 1. After dissolving with a small amount of DMSO, it was dissolved in sterile water for injection, stirred to dissolve, filtered with a sterile suction filter funnel, and then sterile finely filtered. Packed in ampoules, freeze-dried at low temperature and sealed aseptically to obtain powder injection.
[0078] 3. According to the method of Example 1, the compound 1-12 was firstly prepared, and the excipient was added according to the weight ratio of the compound 1-12 to the excipient at a ratio of 9:1 to make a powder.
[0079] 4. According to the method of Example 1, the compound 1-12 was firstly prepared, and the excipient was added according to the weight ratio of the compound 1-12 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com