Fluorescence immunoassay test paper for quantitative joint detection of multiple indexes of ovarian reserve function
A fluorescent immunity and ovarian reserve technology, applied in the field of fluorescent immunoassay test strips, can solve the problems of inability to easily and quickly detect multiple indicators, low degree of automation, radioactive contamination, etc., to achieve reliable detection results, reduce detection costs, Accurate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In the present invention, the sample pad is preferably a whole blood filtration sample pad. The material of the sample pad is preferably a glass cellulose membrane; the preparation method of the sample pad preferably includes the following steps: after soaking the glass cellulose membrane in the blocking solution, dry; the soaking time is preferably 3-7min, more preferably 5min; the blocking solution preferably includes the following components: 0.05wt% Tris, 1wt% tritonX-100, 0.5wt% PEG6000, 0.4wt% bovine serum albumin and 0.02wt% mouse antibody human red blood cells.
[0033]In the present invention, after the binding pad is lapped on the sample pad, the binding pad is sprayed with a fluorescent microsphere-labeled monoclonal antibody mixture; the monoclonal antibody mixture includes the first follicle-stimulating hormone monoclonal antibody, The first luteinizing hormone monoclonal antibody, the first anti-Müllerian hormone monoclonal antibody and the first serum in...
Embodiment 1
[0046] The structure of fluorescent immunoassay paper combined with quantitative detection of multiple indicators of ovarian reserve function
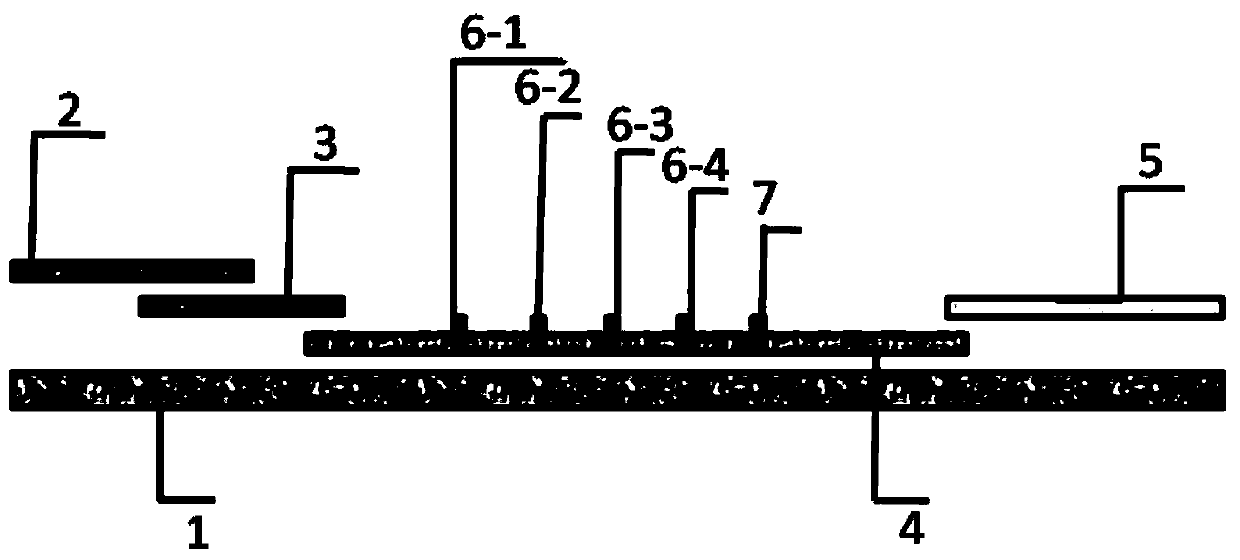
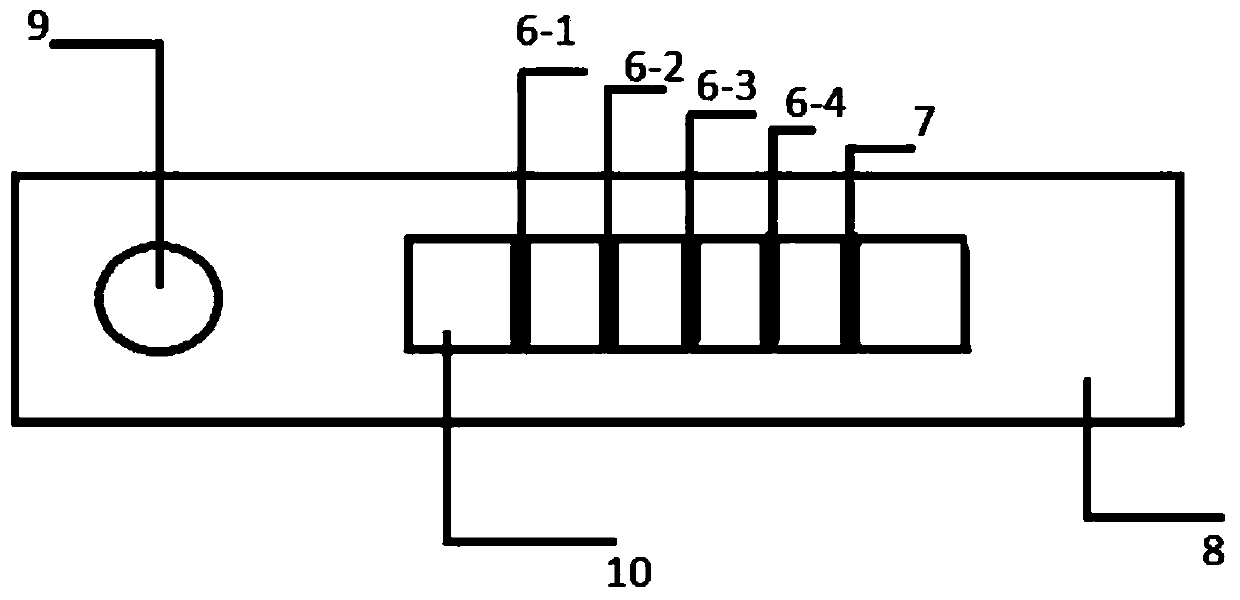
[0047] A fluorescent immunological test strip for combined quantitative detection of multiple indicators of ovarian reserve function. The test strip comprises a sample pad, a binding pad, a nitrocellulose membrane and absorbent paper sequentially connected to a PVC bottom plate. structured as figure 1 Shown; wherein the PVC bottom plate of the test strip is sequentially stuck with nitrocellulose membrane (4), absorbent paper (5), binding pad (3), and a sample pad (2) is laid on the upper layer of the binding pad; Among them, 1mm wide FSH detection line (6-1), LH detection line (6-2), AMH detection line (6-3), INHB detection line (6-4) are drawn in parallel on the nitrocellulose membrane (4). ) and quality control line (7), the distance between each detection line and the quality control line is 8mm; after assembly, cut according to th...
Embodiment 2
[0062] (1) Drawing of fitting standard curve
[0063] Linear dilution of standard antigen: use negative bovine serum to linearly dilute FSH and LH standard antigen: 150mIU / mL, 100mIU / mL, 75mIU / mL, 50mIU / mL, 25mIU / mL, 10mIU / mL, 5mIU / mL, 0mIU / mL; AMH standard antigen was linearly diluted with negative bovine serum: 10ng / mL, 5ng / mL, 2.5ng / mL, 1ng / mL, 0.5ng / mL, 0.25ng / mL, 0.1ng / mL, 0ng / mL mL; linearly dilute INHB standard antigen with negative bovine serum: 2000pg / mL, 1000pg / mL, 500pg / mL, 250pg / mL, 100pg / mL, 50pg / mL, 25pg / mL, 0pg / mL. Add the standard sample to the sample hole of the test paper, use the same batch of test paper and reagents, repeat the measurement for each concentration more than 3 times, use the fluorescence immunoassay analyzer to obtain the fluorescence intensity data of the detection line and the quality control line, analyze the data, and The concentration of the standard is the abscissa, and the fluorescence intensity ratio (T / C) of the detection line and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com