Articular cartilage repair
A technology for cartilage repair and cartilage damage, applied in the direction of joint implants, joint implants, bone implants, etc., can solve the problems of not providing anchoring shape retention features, not including orderly woven matrix, etc., to achieve Effects of Joint Consistency, Maintaining Consistency, Restoring Joint Shape and Function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0072] To promote an understanding of the principles of the invention, reference will now be made to the embodiments described in the following written specification. It should be understood that no limitation of the scope of the invention is intended. It is also to be understood that the invention includes any changes and modifications to the illustrated embodiments, and includes further applications of the principles of the invention which would normally occur to one skilled in the art to which the invention pertains.
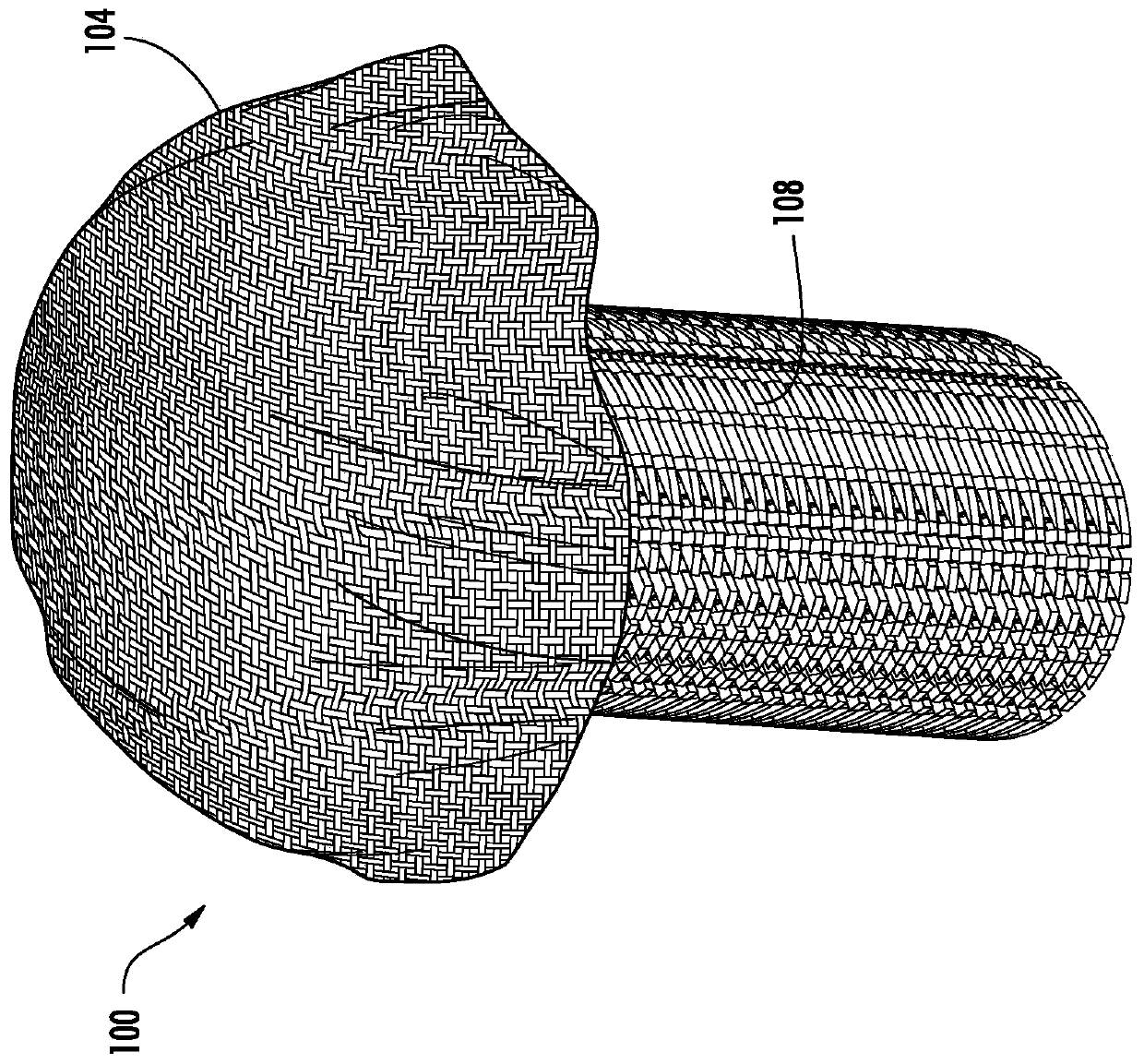
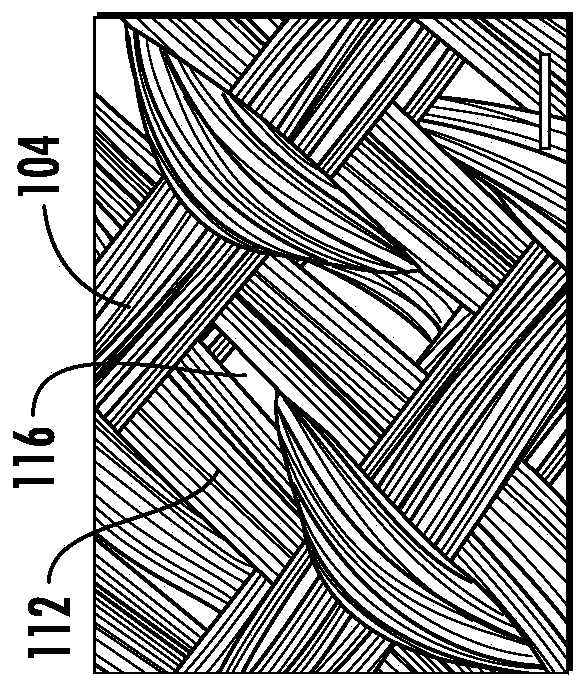
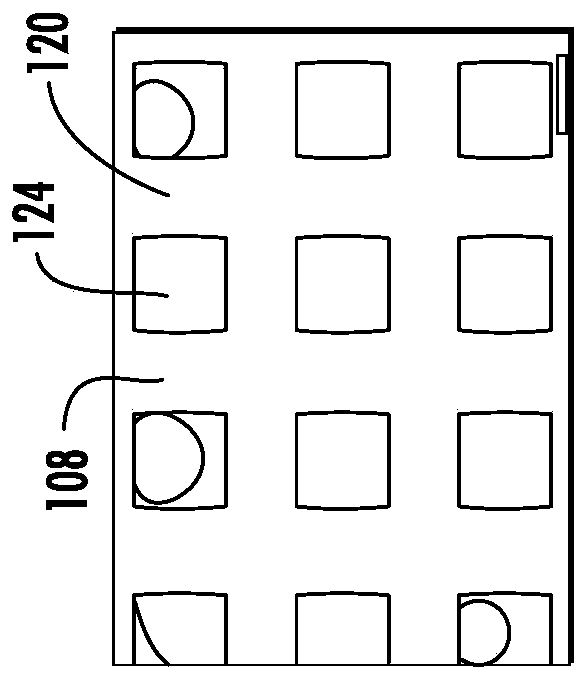
[0073] Most biomaterials used for cartilage tissue engineering do not possess the mechanical and load-bearing properties of native cartilage until significant matrix deposition occurs over time and thus may require significant ex vivo culture prior to implantation. US Patent 8,691,542 discloses a three-dimensional "microbraid" of fibers in three orthogonal directions. The process is superior to standard weaving methods because it eliminates fiber crimp and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compression modulus | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com