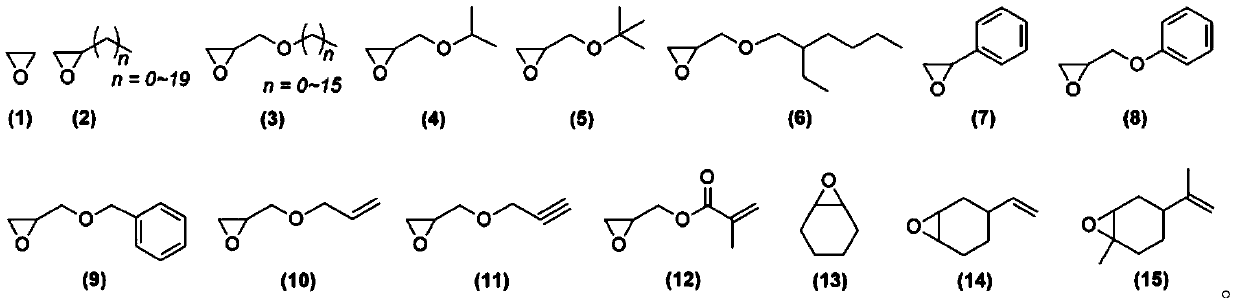
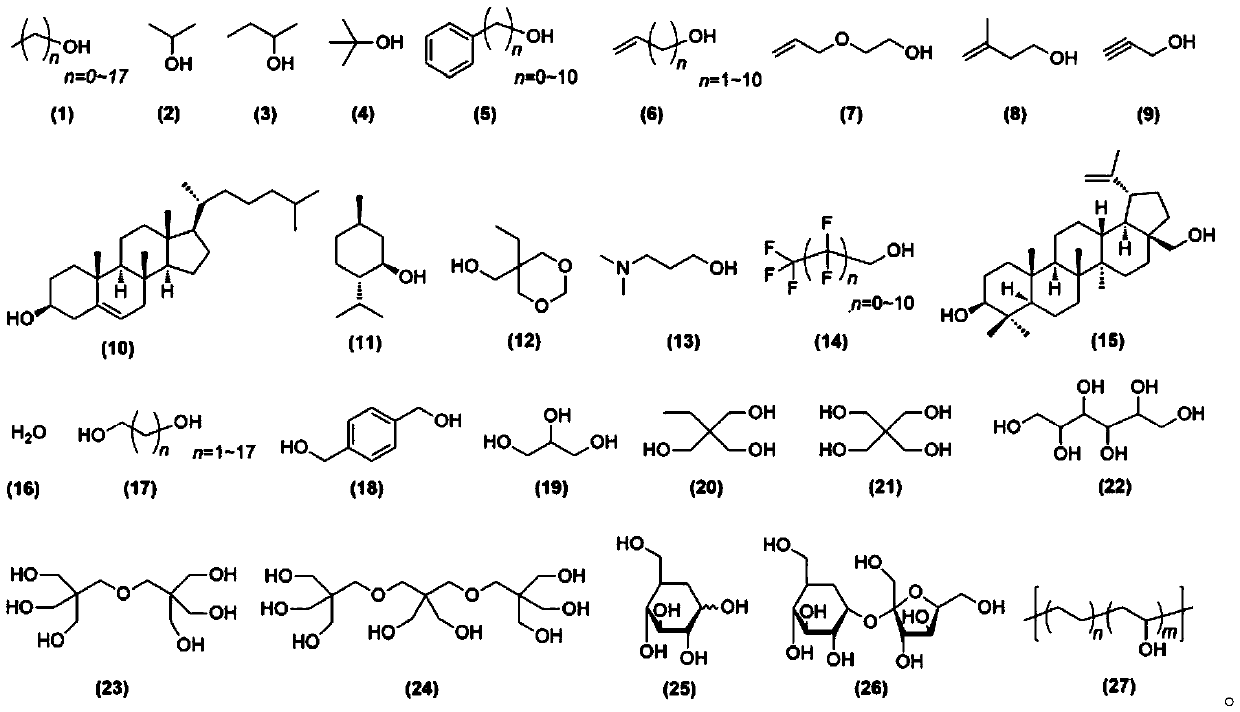
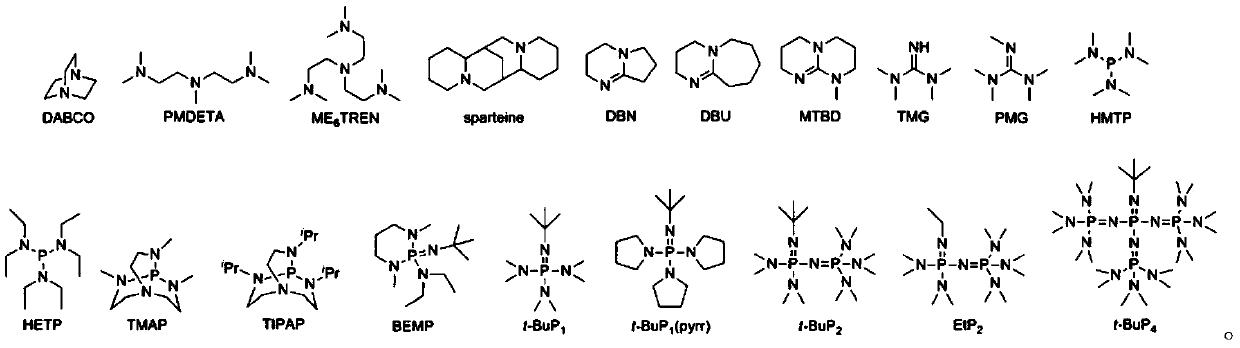
Phthalic anhydride and epoxy compound copolymerization and sequence control method
An epoxy compound and sequence control technology, applied in the field of organic synthesis, can solve the problems of limiting the flexibility of the synthesis method block sequence and the diversity of block copolymer properties, to solve the problem of insufficient polymerization activity and controllability, and simple structure , the effect of simplifying the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In this example, the ring-opening copolymerization of phthalic anhydride and ethylene oxide is carried out by using p-phthalic acid as the initiator and the organic Lewis acid-base pair as the catalyst, and the amphiphilic ether ester block copolymer based on phthalic anhydride and ethylene oxide is prepared in one step . The specific operation is as follows:
[0036] Both tetrahydrofuran (THF) and ethylene oxide were used after water removal treatment. In an inert atmosphere, add 1 part of terephthalic alcohol and 100 parts of phthalic anhydride into a dry glass reactor, and add tetrahydrofuran to dissolve. Continue to add phosphazene base t-BuP containing 1 part 1 With 0.3 parts of tetrahydrofuran solution of triethylboron, stir and mix evenly. Add 1000 parts of dry ethylene oxide, seal the glass reactor and react at room temperature (20-25°C) for 18h. In this embodiment, the molar concentration of ethylene oxide is 7mol / L. After the polymerization reaction, it ca...
Embodiment 2
[0040] In this example, ring-opening copolymerization of phthalic anhydride and ethylene oxide is carried out by using water as an initiator and an organic Lewis acid-base pair as a catalyst, and an alternating copolymer based on phthalic anhydride and ethylene oxide is prepared in one step. The specific operation is as follows:
[0041] Both tetrahydrofuran (THF) and ethylene oxide were used after water removal treatment. In an inert atmosphere, add 1 part of pure water and 50 parts of phthalic anhydride into a dry glass reactor, and add tetrahydrofuran to dissolve. Continue to add t-BuP containing 0.05 parts of phosphazene base 1 With 0.01 parts of triethylboron in tetrahydrofuran solution, stir and mix evenly. Add 50 parts of dry ethylene oxide, seal the glass reactor and react at room temperature for 24h. In this embodiment, the molar concentration of ethylene oxide is 3 mol / L. After the reaction is completed, the reactor is opened, and the copolymerized product is take...
Embodiment 3
[0044] In this example, the ring-opening copolymerization of phthalic anhydride and ethylene oxide was carried out by using terephthalmic alcohol as the initiator and organic Lewis acid-base pair as the catalyst, and a random copolymer based on phthalic anhydride and ethylene oxide was prepared in one step. The specific operation is as follows:
[0045] Both tetrahydrofuran (THF) and ethylene oxide were used after water removal treatment. In an inert atmosphere, add 1 part of terephthalic alcohol and 100 parts of phthalic anhydride into a dry glass reactor, and add tetrahydrofuran to dissolve. Continue to add t-BuP containing 1 part of phosphazene base 1 With 5 parts of tetrahydrofuran solution of triethylboron, stir and mix evenly. Add 500 parts of dry ethylene oxide, seal the glass reactor and react at room temperature for 5h. In this embodiment, the molar concentration of ethylene oxide is 6 mol / L. After the reaction is completed, the reactor is opened, and the copolymer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com